A national multicenter trial — Methotrexate on Effusion-synovitis and Symptoms in Knee Osteoarthritis (MESKO) (MESKO trial) — examining the use of methotrexate for inflammatory knee osteoarthritis (OA) has been published in JAMA Internal Medicine. It was conducted through collaboration between Professor Ding Changhai from Zhujiang Hospital of Southern Medical University and the rheumatology team led by Chief Physician Zeng Xiaofeng and Chief Physician Leng Xiaomei from PUMCH. The study doesn’t support the use of low-dose methotrexate (≤15mg weekly) in patients with inflammatory knee OA, suggesting the need to reassess treatment strategies for knee OA. Professor Nancy E. Lane, Director of the Center for Musculoskeletal Health at UC Davis and Associate Editor of Nature Reviews Rheumatology, published an Invited Commentary that favorably reviewed this research.

Knee OA is one of the most common joint diseases and a leading cause of joint pain, loss of function, and disability among the elderly. There are over 500 million patients worldwide, and this number continues to rise as population ages. Treatment options for knee OA remain severely limited. Methotrexate, a disease-modifying antirheumatic drug and first-line treatment for inflammatory joint diseases, has long been of interest for its potential in treating knee OA, but its efficacy has not been validated by a large-scale clinical trial.
From July 2019 to January 2023, the research team conducted a nationwide multicenter, randomized, double-blind, placebo-controlled investigator-initiated trial (MESKO trial). The study randomized 215 patients and observed them over 52 weeks. It confirmed that the methotrexate group demonstrated no statistically significant difference in knee pain or effusion-synovitis on magnetic resonance imaging from the placebo group, establishing methotrexate’s lack of efficacy in treating knee OA. The findings highlight the urgent need to identify more effective alternative therapies.
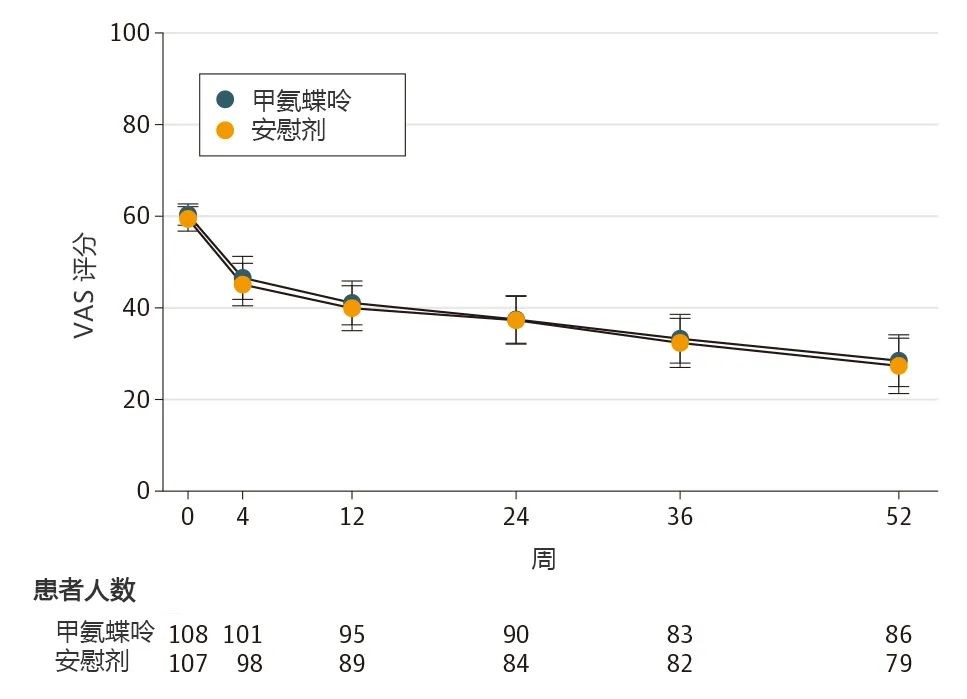
▲Research results
Subgroup analysis of patients with severe pain (baseline pain scores ≥80mm) suggested that methotrexate might provide more significant symptom relief for these patients. However, given the small sample size in this subgroup, these results should be interpreted with caution. This also indicates the necessity for future clinical trials specifically targeting this subgroup.
The study has garnered significant attention from the academic community. Professor Nancy E. Lane, Director of the Center for Musculoskeletal Health at UC Davis, Associate Editor of Nature Reviews Rheumatology, and President-Elect of the Osteoarthritis and Cartilage Research Society International (OARSI), published an Invited Commentary in JAMA Internal Medicine. Referencing the MESKO trial, Professor Lane analyzed the possible mechanisms underlying methotrexate's lack of efficacy in knee OA. She argued that precision medicine is essential for advancing knee OA treatment and called for accelerated drug development.

▲Invited Commentary by Professor Nancy E. Lane
Chief Physician Zeng Xiaofeng is the co-corresponding author, and Chief Physician Leng Xiaomei the co-first author.
Written by and pictures courtesy of the Department of Rheumatology
