Recently, a prospective, multicenter, randomized controlled study led by Professor Xiang Yang, Director of the Gynecological Oncology Center at PUMCH, was published in the internationally renowned journal Annals of Oncology. The study compared two first-line single-agent chemotherapy for gestational trophoblastic neoplasia (GTN), examining differences in such aspects as medication regimens, adverse events, and patient experience. This provides clearer guidance for individualized treatment across different patient populations and offers evidence for updating international guidelines, showcasing China's research strength and global impact in proper GTN care. The study was supported by the National Key R&D Program of China and the National High Level Hospital Clinical Research Funding, among others.

GTN is a group of malignant tumors derived from placental trophoblastic cells that are highly chemotherapy-sensitive. International guidelines recommend single-agent chemotherapy as first-line treatment for low-risk GTN patients. The two most widely used agents are methotrexate (MTX) and actinomycin D (Act-D). These drugs differ in medication regimens, adverse events, and patient experience. Recommended options vary across countries and guidelines. For instance, the International Federation of Gynecology and Obstetrics (FIGO) favors Act-D, while the National Comprehensive Cancer Network (NCCN) guidelines prefer MTX.
Previous studies on single-agent chemotherapy for low-risk GTN have been limited by small sample sizes, single-center designs, or retrospective analyses, with study populations predominantly from Europe and America. High-quality prospective evidence for Asian populations has been lacking. There was an urgent need for a large-scale, prospective, multicenter randomized controlled trial comparing MTX vs. Act-D efficacy and safety in low-risk GTN. This would provide evidence-based guidance for more precision clinical decision-making, improve treatment outcomes, and support patients' long-term reproductive health.
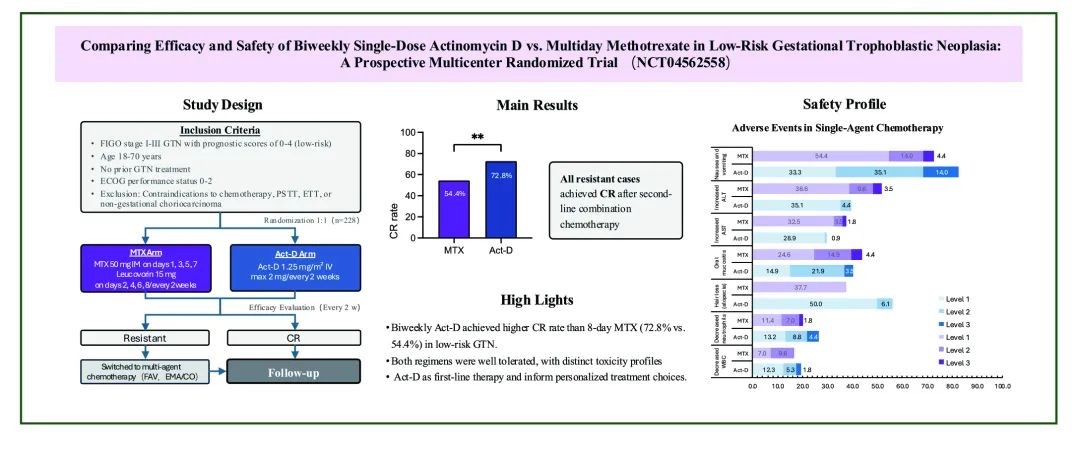
▲Study design and main results
This five-year collaborative study was led by Professor Xiang Yang's team in partnership with multiple GTN care centers nationwide. The trial enrolled 228 patients with low-risk GTN (FIGO scores 0-4), randomized to receive either the 8-day MTX regimen or biweekly Act-D therapy. Results demonstrated that Act-D achieved significantly higher single-agent complete remission rates than MTX (72.8% vs. 54.4%, P=0.0038). All patients resistant to single-agent therapy achieved complete remission after second-line combination chemotherapy. Among responders, Act-D achieved remission in a shorter time than MTX, which helps ease patient anxiety. While overall adverse event rates were similar between groups, the toxicity patterns differed: Act-D was associated primarily with mild gastrointestinal symptoms and alopecia, whereas MTX was associated with higher rates of mild liver dysfunction.
Long-term follow-up is ongoing for all enrolled patients. This research provides high-quality evidence for optimizing treatment decisions and has garnered significant international attention. The oral presentation on the findings was recognized as a new breakthrough presentation at the 25th Society of Gynecologic Oncology (SGO) Annual Meeting in March 2025.
Professor Xiang Yang, Director of the Gynecological Oncology Center at PUMCH, is the corresponding author, and Dr. Jiang Fang, Chief Physician of the Gynecological Oncology Center, is the first author.
Written by Jiang Fang and Xiang Yang
Pictures courtesy of the Department of Obstetrics and Gynecology
Edited by Fu Tanping and Chen Xiao
