The research team led by Director Han Xiaohong and Assistant Director Zheng Xin of the Clinical Pharmacology Research Center at PUMCH, in collaboration with the team led by Deputy Director Ma Mingsheng of Pediatrics and researchers from China University of Mining and Technology (Beijing), have developed Derivatization-Enhanced EIEIO-Driven Glycosidic Linkage Sequencing (DEED-GL-Seq), a novel platform for oligosaccharide linkage analysis.
This breakthrough tackles longstanding challenges in analyzing glycan structures and shows promising results for diagnosing Pompe disease (Glycogen storage disease type II, GSD-II), a rare genetic disorder. The research was recently published in Carbohydrate Polymers, a leading international journal in glycochemistry and glycomics.
Sugars are not only sources of energy but also important regulatory factors of cellular function. Complex sugar molecule chains, such as oligosaccharides, in the human body are like strings of tiny codes carrying important biological information closely related to health. However, decoding these "sugar codes" is extremely difficult because sugar structures are far more variable and complex than biological polymers like proteins and nucleic acids. Different oligosaccharide isomers may reflect different biological functions. Structural analysis of carbohydrates has always been challenging. Traditional analytical methods are inefficient and costly, resulting in very limited understanding of oligosaccharide biological functions.
In this study, the research team cleverly combined "derivatization" with "EIEIO (electron-induced excitation of ionized organics) fragmentation", a high-energy electron fragmentation technique, to create a new platform for analyzing complex oligosaccharide glycosidic linkages: DEED-GL-Seq. This integration of chemical modification and advanced fragmentation technology is a major breakthrough that enables precision "decoding" of complex glycan structures.
The research team first applied a hydrazine-based derivatization reagent (T3) to attach "tags" to key connection points (glycosidic bonds) in the oligosaccharides, significantly amplifying subtle differences between different linkage types. They then used EIEIO to fragment the oligosaccharides. Because the bonds had been pre-labeled, the resulting fragments carry distinct "identity information" that clearly reveals the original connection patterns and sequences of glycans.
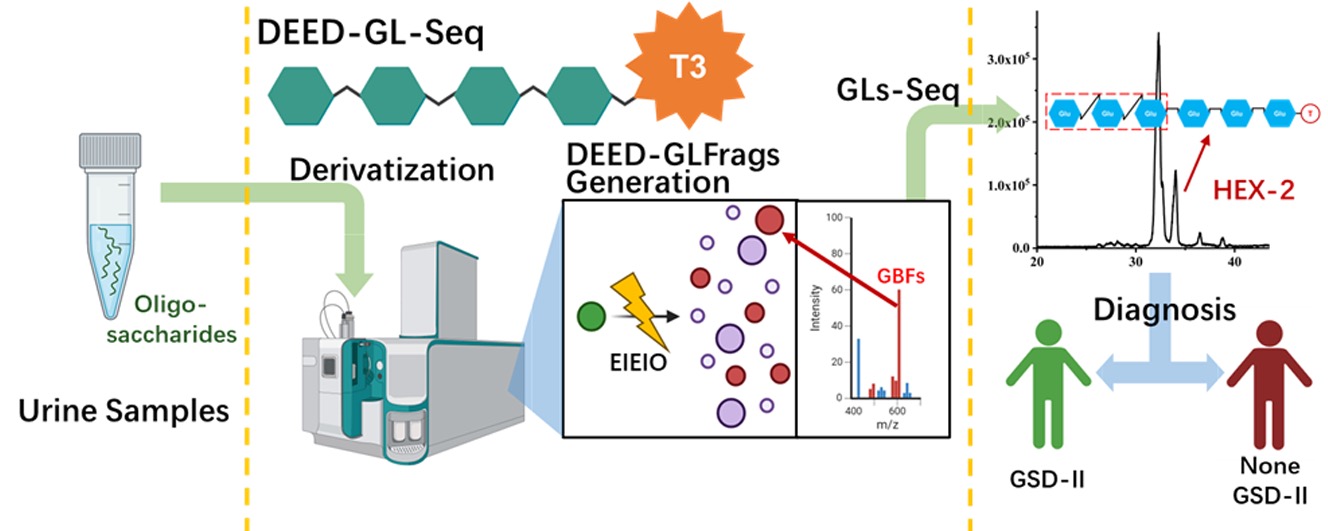
▲DEED-GL-Seq workflow
The research team applied this new platform to urinary oligosaccharide analysis in patients with Pompe disease, precisely identifying new "fingerprints" for the disease.
Pompe disease is a rare hereditary glycogen storage disease: patients lack an enzyme that breaks down glycogen, causing abnormal glycogen accumulation in muscles and other tissues that leads to severe symptoms. Using DEED-GL-Seq, the team precisely analyzed the structure of the Pompe disease-related oligosaccharide biomarker (Glc₄) reported in literature; more importantly, they discovered and accurately identified for the first time four novel potential glycosidic biomarkers (HEX-1, HEX-2, HEPTA-1, HEPTA-2) for Pompe disease. Among them, the hexasaccharide molecule HEX-2 stood out. The team thus speculated that it originates from abnormal metabolic pathways specific to Pompe disease. HEX-2 shows very low correlation with other known biomarkers but demonstrates extremely strong specificity for Pompe disease, holding promise as a new biomarker for precision diagnosis of Pompe disease.
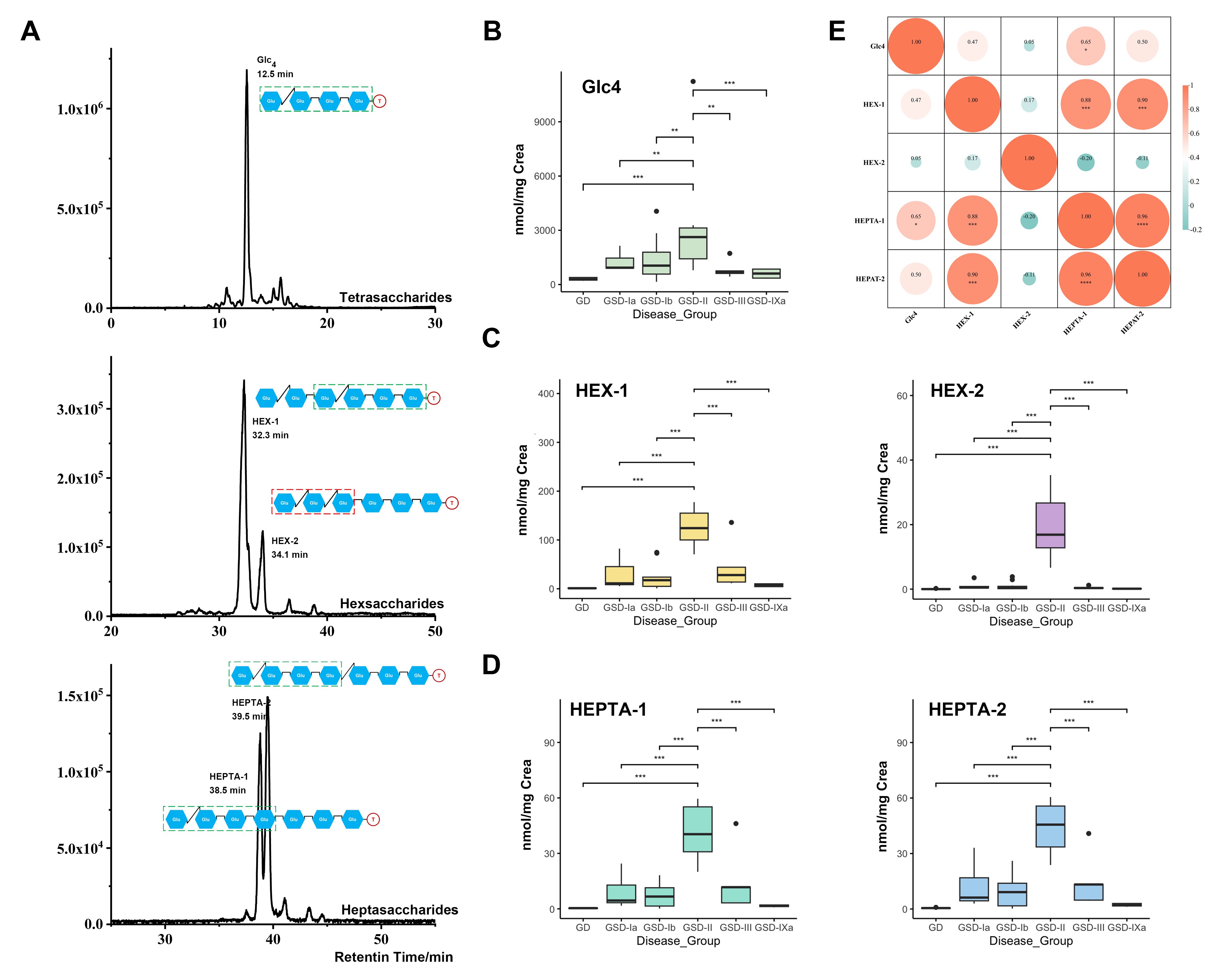
▲Five Pompe disease oligosaccharide biomarkers identified in urine from patients with different subtypes of glycogen storage diseases, with HEX-2 showing extremely strong specificity for Pompe disease
This platform overcomes the challenges of complex glycan structural analysis and reduces reliance on expensive reference standards, enabling cost-effective large-scale sequencing of intricate glycan structures. According to Dr. Han Xiaohong, the platform not only offers new diagnostic tools and therapeutic targets for Pompe disease, but also shows great promise for supporting biomarker discovery and precision medicine approaches for numerous other diseases involving glycometabolic disorders. A national invention patent has been filed for the related technology.
Dr. Han Xiaohong and Dr. Ma Mingsheng are co-corresponding authors of this paper, while Dr. Zheng Xin, Senior Technician Ma Yufang from the Clinical Pharmacology Research Center, and Assistant Researcher Cui Xinge from the Stem Cell Facility under the Institute of Clinical Medicine are co-first authors.
Written by Wang Lu
Pictures courtesy of Zheng Xin
Edited by Wang Lu
Chief Editor Duan Wenli
Supervised by Wu Peixin