A team led by Dr. Zhang Wen, Deputy Director of Rheumatology at PUMCH, recently published their research findings in Science Bulletin. Their study demonstrates for the first time that overexpression of early growth response-1 (EGR1) in epithelial cells is a core driving factor of the characteristic tissue fibrosis seen in IgG4-related disease (IgG4-RD), laying the scientific groundwork for potential targeted therapies. The research was funded by China's National Key R&D Program during the 14th Five-Year Plan Period, the National Natural Science Foundation of China, and the National High Level Hospital Clinical Research Funding, among others.

IgG4-RD is a chronic immune-mediated inflammatory disorder marked by multi-organ swelling and induration, extensive lymphocyte and plasma cell infiltration in affected tissues, and prominent fibrosis. The disease can affect critical organs including the pancreas, lacrimal glands, salivary glands, retroperitoneum, lungs, and kidneys. Conventional treatments often lead to relapse. While previous studies focused on immune cell dysfunction, this research took an innovative approach by analyzing single-cell RNA sequencing (scRNA-seq) data from IgG4-RD patients and conducting cellular model experiments, shifting the focus to epithelial cells within affected tissue.
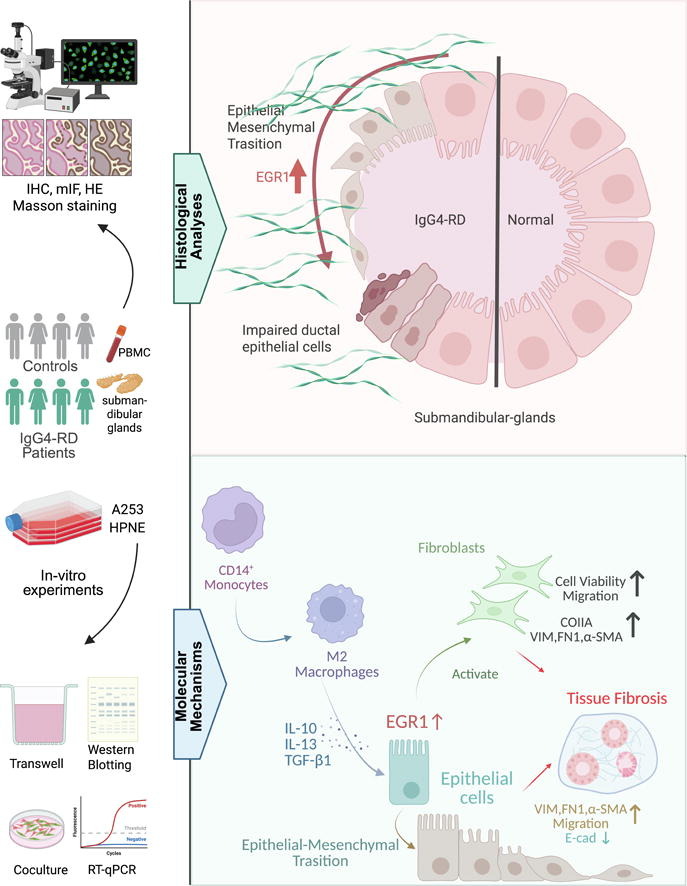
The team discovered significantly elevated EGR1 expression in epithelial cells from affected tissues of IgG4-RD patients compared to normal tissue. More importantly, these EGR1-overexpressing epithelial cells exhibited distinct features of epithelial-mesenchymal transition (EMT). This transformation process directly enhanced the migratory mobility of epithelial cells, serving as a critical starting point for abnormal tissue remodeling.
The research demonstrated that EMT-positive epithelial cells activate fibroblasts—the primary fibrotic effector cells—through secretion of specific factors, promoting fibroblast proliferation, migration, and expression of fibrotic markers. Using submandibular gland and pancreatic epithelial cell models with engineered EGR1 overexpression and knockdown, researchers showed that EGR1 overexpression successfully induced expression of mesenchymal and fibrotic markers including VIM, FN1, and α-SMA, while significantly boosting migratory mobility. Culture supernatant derived from EGR1-overexpressing epithelial cells effectively stimulated fibroblast proliferation and migration while upregulating fibrosis-associated marker expression.
▲Research schematic diagram
Through co-culture experiments, the researchers mapped out a clear pathogenic cascade, identifying EGR1 as a central signaling hub. They confirmed that cytokines released by activated CD14+ monocytes and their M2 macrophage derivatives in the disease microenvironment upregulate epithelial EGR1 expression, subsequently triggering EMT. EGR1 functions as the pivotal signaling node linking immune activation, epithelial cell transformation, and fibroblast activation in this pathogenic cascade.
Dr. Zhang Wen, Chief Physician at PUMCH, and Associate Researcher Wu Xunyao are co-corresponding authors. Ph.D. candidates Sun Ruijie and Luo Qinhuan as co-first authors.
Written by Sun Ruijie, Luo Qinhuan, Zhang Wen and Wu Xunyao
Pictures courtesy of the Department of Rheumatology
Edited by Fu Tanping and Chen Xiao
Chief editor Duan Wenli
Supervised by Wu Peixin
