China has made remarkable strides in rare disease management in recent years. What was once a landscape where "doctors who can treat rare diseases are rarer than the diseases themselves" has transformed dramatically. Diagnosis time has been reduced from 4 years to just 4 weeks. Treatment options have evolved from "no available medication" to medications that are not only accessible but also affordable, with more than 90 medications for rare diseases now included in the National Medical Insurance Drug Catalog. The establishment of China's first State Key Laboratory for Complex, Severe and Rare Diseases and the deployment of the country's first AI model for rare diseases have further enhanced these efforts. Through these endeavors, China has made diagnosis and treatment much more standardized, established a comprehensive multi-tiered medication access system, and accelerated the pace of clinically oriented translation of innovative outcomes.
Over the past six years, PUMCH has, from the ground up, created a "PUMCH model" for rare disease diagnosis, treatment, and research with a "patient-centered" approach, leading the development of "Chinese solutions" that align with international standards and stand at the global forefront. Each "impossible" breakthrough reflects PUMCH staff's firm commitment to safeguarding public health and the profound respect for life shared by both medical professionals and patients.

▲On February 28, 2025, International Rare Disease Day, PUMCH held a free clinic activity
Navigating Diagnostic Challenges to Find Paths Through the "Maze"
"This patient is unfortunate, but she's lucky to have come to PUMCH." This comment from Professor Ye Hongying of the Department of Endocrinology and Metabolism at Huashan Hospital, Fudan University during a rare disease MDT (multidisciplinary team) consultation echoes the sentiments of many rare disease patients.

▲In the noontime of February 27, 2025, the 281st rare disease MDT consultation was held
A 19-year-old girl had suffered from sparse hair, mottled skin, hematuria, proteinuria, and lower limb edema after exercise or fatigue since childhood, but the cause remained elusive. In 2023, following a rare disease MDT consultation at PUMCH, she was diagnosed with Hypotrichosis-Lymphedema-Telangiectasia Syndrome (HLTS). She became the first confirmed case of this rare disease in China and the 16th worldwide.
When she was discharged from PUMCH for the second time in July 2024, she wrote: "May you take good care of yourselves amid your busy work; may you find your own happiness and joy while protecting others' health!" Her graceful handwriting conveys not only the upbeat girl's unyielding spirit in the face of tough challenges but also the courage of rare disease patients and doctors fighting side by side.

▲The HLTS patient's heartfelt message to medical staff before her discharge
On February 28, 2019, the International Rare Disease Day six years ago, PUMCH established the country's first national-level rare disease consultation platform—the PUMCH Rare Disease MDT Platform. Over the past six years, that MDT platform has received 279 patients.
From the highly satisfying model of "multiple specialists serving one patient" to the highly efficient model of "multiple specialists serving multiple patients", rare disease diagnosis and treatment efficiency saw a leapfrogging improvement in 2023.

▲Group photo of the Department of Rare Diseases
In April 2023, PUMCH established the Department of Rare Diseases and opened a joint outpatient clinic for rare diseases. On its first day, the clinic received 29 patients. Patients could be referred to multiple specialized departments on the day of consultation, benefiting from the "one-stop" solution. To date, 57 specialists from 21 departments have provided services at the joint clinic for rare diseases.

▲Some specialists posed for a group photo on the inauguration day of the joint outpatient clinic for rare diseases on April 10, 2023
A year later, the first dedicated rare disease ward in China began operation on the International Rare Disease Day. In the past year, the ward has treated 329 patients, with a diagnosis rate of nearly 60% and a patient satisfaction score of 100.

▲Hospital leaders visiting the first batch of patients admitted to the Department of Rare Diseases
In just five years, PUMCH has developed a collaborative multidisciplinary approach to rare diseases that integrates outpatient services, inpatient care, and specialist consultations into a cohesive system. These efforts set in motion a “closed-loop” care pathway for rare disease patients, yet for the PUMCH team, this was merely the beginning.

▲In early 2025, research teams joined ward rounds by the Department of Rare Diseases
On the afternoon of December 31, 2024, and the morning of January 4, 2025, at the turn of the year, two special ward rounds marked an upgrade in PUMCH's multidisciplinary collaborative model for rare diseases. The ward rounds in the new year were joined by "new faces"—researchers from the State Key Laboratory for Complex, Severe and Rare Diseases, Institute of Clinical Medicine, and National and Beijing Rare Disease Quality Control Centers. A week later, a "Clinical-Genetic-Basic" case discussion was held, where clinicians, geneticists, and basic research scientists worked together to unravel the mysteries and find diagnostic and treatment clues for diseases. "Rare disease patients are primarily children and adolescents; we have a long way to go", said Professor Shen Min, Director of the Department of Rare Diseases.
The deep integration of clinical practices and scientific research is the necessary path to breakthrough in rare disease diagnosis and treatment methods. The fully integrated "diagnosis-treatment-research" mechanism gives the "PUMCH solution" for rare disease diagnosis and treatment more substance.
When expertise from multiple disciplines converges, even the most perplexing diagnostic mysteries begin to unravel. Through their commitment to excellence, PUMCH's healthcare professionals continue to guide patients through the labyrinth of rare diseases toward clearer understanding and better care.
Striving against All Odds towards the Beacon of Hope
While arriving at a clear diagnosis represents just the first step in the long and arduous journey, many rare disease patients find themselves unable to progress further due to limited treatment options. "The suffering of these patients and our limitations in treating them weigh heavily on each of us", explained Professor Zhang Shuyang, President of PUMCH and Director of the Rare Disease Medical Center. "Though medicine has its boundaries, we as physicians can and must push those boundaries to open up new possibilities".
Ms. Xie had been suffering from intravenous leiomyomatosis for more than two years. Just as she was about to undergo surgery, she unexpectedly discovered she was pregnant. Although it is a benign tumor, this rare disease is extremely invasive and can even lead to death. Ms. Xie and her family's wish to keep the baby completely contradicted domestic and international guidelines and experience—it seemed a "luxury". Faced with this highly challenging situation, the medical staff rose to the occasion.
On April 28, 2024, under the guidance of Academician Lang Jinghe, a team of experts led by Dr. Fan Qingbo, Chief Physician of the Department of Obstetrics and Gynecology, along with Deputy Director Chen Yuexin of the Department of Vascular Surgery, Deputy Director Pei Lijian of the Department of Anesthesiology, Head Nurse Wang Huizhen of the Operating Room, and Deputy Chief Physician Ma Guotao of the Department of Cardiac Surgery, successfully completed a "resection of giant pelvic intravenous leiomyoma and removal of inferior vena cava and bilateral iliac vein leiomyoma involving the heart" for Ms. Xie. She was the first patient in the world to undergo this surgery during mid-pregnancy. After the surgery, her pregnancy continued.
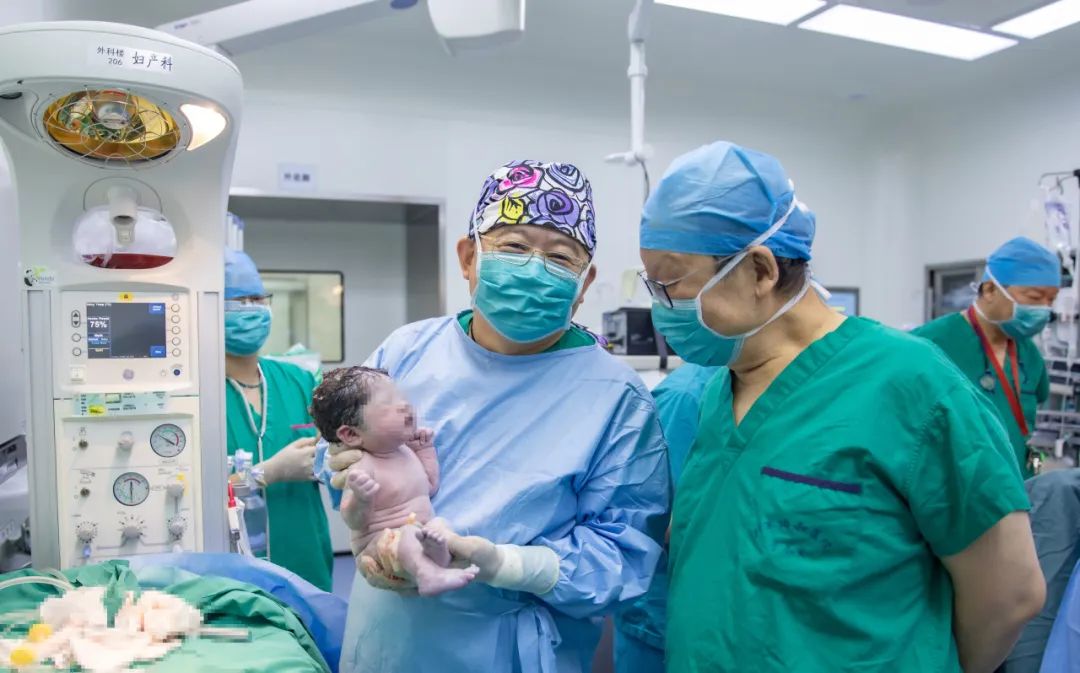
▲On October 8, 2024, Ms. Xie successfully gave birth to a healthy full-term baby at PUMCH. Academician Lang Jinghe (right) and Chief Physician Fan Qingbo (left) welcomed the new life to the world together
Both mother and baby are healthy. This new life stands as the greatest reward for the PUMCH medical team that dared to challenge what was thought impossible
"Taking on cases others turn away from, performing procedures others cannot master"—this confidence to venture into uncharted medical territory is deeply rooted in PUMCH's institutional DNA and legacy of tackling seemingly insurmountable challenges. For these medical professionals, confronting daunting challenges has become second nature.
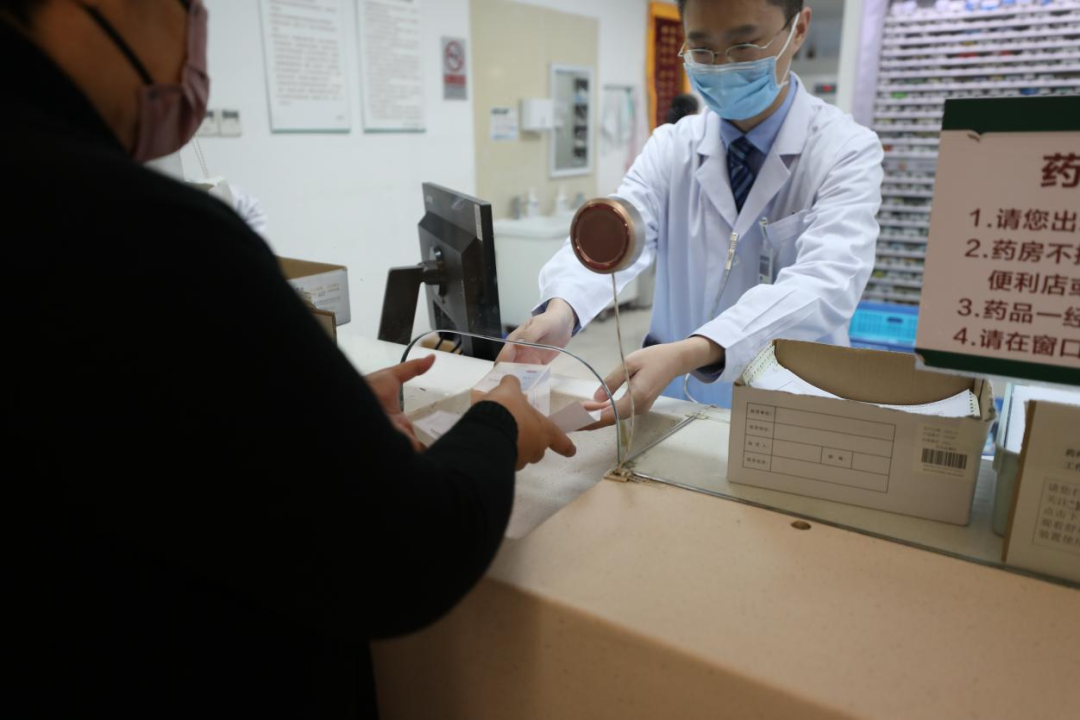
▲On January 9, 2025, PUMCH issued mainland China's first prescription for mitotane, a treatment for adrenocortical carcinoma. The photo shows the first patient receiving the medication
At 1:30 p.m. on January 9, 2025, after a detailed consultation, Professor Zhang Yushi, Director of the Department of Urology at PUMCH, wrote a prescription for the "life-saving drug" mitotane for Ms. Liu, a patient with adrenocortical carcinoma. This was the first prescription issued for mitotane, a treatment for adrenocortical carcinoma, after its launch in mainland China. From market launch to patients receiving the medication, the process took less than 48 hours. With favorable policies that enable expedited approval, PUMCH staff leveraged the "PUMCH speed" to ensure that more patients can receive the "life-saving drug" as quickly as possible.
Turn the clock back to six years ago: this medication was first imported in 2019 by PUMCH through a special rare disease medication channel on a one-time basis. At that time, to address the medication needs of rare disease patients in China, PUMCH carefully prepared and submitted an application to regulatory authorities. They subsequently received approval to import a small quantity of mitotane tablets from abroad as a one-time special import, specifically for patients with adrenocortical carcinoma.
“It was like forging a new path along a steep cliff", said Professor Zhang Bo, Director of the Department of Pharmacy. The medical community described this initiative as "a groundbreaking step for rare disease medications entering China". "The hopes of rare disease patients rest with you!" said Li Linkang, Executive Director of the China Alliance for Rare Disease, expressing his sincere expectations to young physicians before the opening of PUMCH's first Young Physicians' Rare Disease Case Report Competition. PUMCH staff are determined to live up to this expectation.

▲On February 27, 2025, PUMCH held its first Young Physicians' Rare Disease Case Report Competition
In recent years, PUMCH has continuously worked to develop a specialized medication access framework for rare diseases, bringing hope to patients through a series of innovative advances.

▲On May 13, 2024, a ceremony for the temporary import of Defal, a clinically urgently needed drug for the rare disease Duchenne Muscular Dystrophy (DMD), was held at PUMCH. On that day, eight pediatric patients from all over the country received the medication
In 2021, PUMCH addressed the medication access challenges for patients with paroxysmal nocturnal hemoglobinuria through expanded compassionate use programs; in 2022, it issued the country's first prescription for clobazam, a drug for refractory epilepsy; in 2024, it wrote China's first prescription for Defal, a clinically urgently needed drug for Duchenne Muscular Dystrophy (DMD); in October of the same year, PUMCH was among the first batch of hospitals on the “white list of the rare disease medication access pilot zone”; the hospital has facilitated the market launch of 116 medications for 53 rare diseases, reducing drug costs by an average of over 50%.
PUMCH has transformed the landscape for rare disease patients—from a void where treatments didn't exist, to creating access to vital medications, and finally to making these treatments more affordable to patients. Through innovative approaches and unwavering dedication, the hospital has written countless success stories, turning medical impossibilities into life-changing realities for patients.
Embracing Change, Developing New Quality Productive Forces
The true essence of innovation lies in illuminating what was previously hidden in darkness.
On February 16, 2025, the "PUMCH-Genesis" rare disease model, jointly developed over two years by PUMCH and the Institute of Automation, Chinese Academy of Sciences, officially began clinical use. When patients input their symptoms in the dialog box, within seconds, the model makes a preliminary judgment and provides professional advice on which department to visit and what additional examinations are needed. It can even accurately recommend doctors. The professional version can help doctors identify and diagnose rare diseases more accurately and quickly.

▲On February 16, 2025, China's first rare disease model "PUMCH-Genesis" officially began clinical use
The model is built upon PUMCH's extensive collection of high-quality rare and complex disease cases, expert evidence-based decision processes, and multidisciplinary clinical reasoning. Backed by China's comprehensive research data and educational databanks on rare diseases, this system enhances physicians' ability to recognize and diagnose rare conditions efficiently, significantly reducing diagnostic delays. It addresses the longstanding challenge of inconsistent rare disease care across different regions of China. The development of this technology places China at the cutting edge of AI applications in rare disease management globally and represents a significant advancement in China's approach to rare disease diagnosis and treatment.
A year ago, on the 17th International Rare Disease Day, the PUMCH Innovation and Development Institute for Rare Disease Diagnosis and Treatment was established. "PUMCH-Genesis" debuted on this day.
Care to rare disease patients is a "tough nut" that PUMCH staff must crack. At the inauguration ceremony of the PUMCH Innovation and Development Institute for Rare Disease Diagnosis and Treatment, President Zhang Shuyang stated that PUMCH aims to collaborate with various stakeholders to establish cutting-edge innovation platforms, powerful industry clusters, exceptional talent teams, and a thriving innovation ecosystem. These efforts will facilitate effective medical translation, bring forth the new quality productive forces at a faster pace, and bring fresh momentum to healthcare development initiatives.
Now, with "PUMCH-Genesis" officially in clinical use, it is expected to become the "key to solving" the difficulty in diagnosing rare diseases.
Scientific research breakthroughs in the field of rare diseases extend beyond the laboratory.
A 17-year-old patient with Gitelman syndrome struggled with having to take 36 potassium and magnesium tablets daily. The team led by Professor Chen Limeng, Director of the Department of Nephrology, collaborated with pharmaceutical companies to develop microsphere controlled-release tablets with larger dosages and better taste. The project has entered the national drug review priority channel. "We not only want to treat the disease but also want patients to live with dignity." Chen Limeng's goal is also a reflection of the compassion underlying PUMCH's research endeavors.
Discovering problems in clinical practice and then conducting valuable research based on these observations has always been the relentless pursuit of PUMCH staff.

▲On September 27, 2023, the State Key Laboratory of Complex, Severe and Rare Diseases (hereinafter referred to as the State Key Laboratory) was inaugurated at PUMCH
In 2020, the hospital was approved to establish the State Key Laboratory of Complex, Severe and Rare Diseases, providing strong support for accelerating research on rare diseases. They established a new diagnostic method for transthyretin cardiac amyloidosis, replacing invasive procedures with non-invasive ones, increasing the diagnosis rate from 20% to 80%. The hospital led a national multi-center Phase IV clinical trial of tafamidis for ATTR treatment, advancing the drug's domestic marketing and reducing mortality by 70%. They pioneered four renal function tests in China, significantly improving the clinical diagnosis rate of hereditary renal tubular diseases. They developed an AI genetic analysis system for rare diseases with independent intellectual property rights, significantly shortening genetic interpretation time. And the list goes on.
The steady stream of breakthrough research findings moving from laboratory to clinical application gives rare disease patients both faster access to treatment options and greater reason for optimism.

▲China's National Rare Disease Registration System (NRDRS)
With guidance from the National Health Commission, PUMCH spearheaded the development of China's Rare Disease Case Reporting System. Drawing on nearly 800,000 reported cases nationwide, the hospital released the country's first comprehensive mapping of rare disease distribution and treatment resources—a crucial tool for multi-dimensional prevention, treatment, and monitoring of rare diseases. Supported by China's National Key Research and Development Program during the "13th Five-Year Plan", the China Rare Diseases Registry System led by PUMCH now encompasses nearly 200 disease cohorts. Its extensive databases and biobanks have facilitated over 100 clinical trials, elevating China's rare disease information system to global prominence alongside America's NORD and Europe's Orphanet registries.
On the eve of this year's International Rare Disease Day, Professor Zhang Shuyang, as a member of the RDI (Rare Disease International)-Lancet Commission on Rare Diseases, published a comment in the international top journal The Lancet together with other members, explaining the opportunities and challenges facing rare disease diagnosis, treatment, and research, and calling on all stakeholders around the world to take action to accelerate the advancement of rare disease care.


▲Wu Xiaoqi's hand-drawn illustration
A young doctor at the Department of Rare Diseases made a touching drawing for one of their pediatric patients. The artwork depicted "a child trapped by illness, standing alone on a barren world. On this isolated planet, only a few small flowers bloom, nurtured by the devoted care of family members doing everything in their power. Yet I believe that through the joint efforts between medical teams and patients, we can transform this landscape with hopeful sunflowers, giving this child the chance to grow up and be healthy and happy!" It's because of dedicated individuals like this that even the most distant dreams become achievable and the toughest challenges conquerable.

Written by Chen Xiao
Photographed by Sun Liang
Editors Chen Xiao and Xiao Xiong
Chief Editor Duan Wenli
Supervised by Wu Peixin
