Recently, a collaborative study led by Professor Leng Jinhua's team from the Department of Obstetrics and Gynecology and Director Wang Xiaoyue's team from the Bioinformatics Technology Platform at Peking Union Medical College Hospital (PUMCH) was published in Cell Genomics (IF=11.1). The research elucidates the molecular characteristics of ectopic endometrial cells and their interactions within the lesion microenvironment, offering potential avenues for early diagnostic biomarkers and tailored therapeutic strategies for diverse subtypes of endometriosis. This work was supported by the National High-Level Hospital Clinical Research Funding and other grants.

Endometriosis is a common gynecological condition affecting approximately 10% of women of reproductive age, is often termed as a “benign cancer” due to its malignancy-like behavior. It occurs when endometrial tissue, which normally lines inside of the uterus and sheds during menstruation, grows abnormally elsewhere—such as on the peritoneal surfaces, ovaries, uterosacral ligaments, and outer surface of the uterus. Current treatments, including surgical excision and hormonal suppression, often cause significant side effects and fail to provide long-term remission, functioning as temporary fixes rather than addressing the root cause.
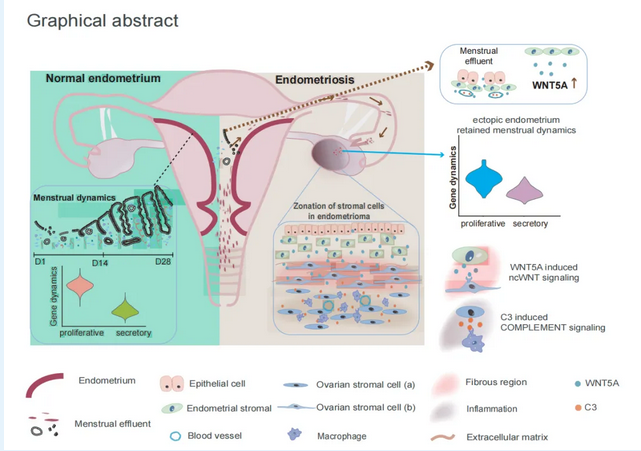
To investigate disease mechanisms, the research team collected eutopic endometrium and ectopic lesions from endometriosis patients (with ovarian endometriomas), as well as eutopic endometrium from non-endometriosis controls. Leveraging single-cell sequencing and spatial transcriptome sequencing, they mapped the molecular profiles of ectopic lesions and endometrial tissues..
▲Schematic of ovarian endometriotic lesion microenvironment: WNT5A-positive ectopic endometrial stromal cells accumulate in ovarian cyst lesions and modulate the microenvironment via non-canonical WNT(ncWNT) signaling
The study identified distinct populations of ectopic endometrial stromal (EnS) cells and ovarian stromal cells (OSCs) . Despite being displaced, ectopic EnS cells retained hormonal responsiveness akin to normal endometrial cells, yet exhibited unique gene expression linked to disease progression. OSCs were categorized into two functional subsets: one acting as an "inflammation commanders" that recruit immune cells, and the other functioning as "fibrotic builders" that secrete extracellular matrix to encapsulate lesions. These cells formed a three-layered architecture—ectopic EnS cells encased by fibrotic builders, with inflammatory commanders forming the outermost layer.
Crucially, the WNT5A signaling pathway was found to drive crosstalk between ectopic EnS cells and OSCs. Ectopic EnS cells manupulate this pathway to send aberrant growth signals to neighboring OSCs, acting as a molecular "trigger switch" that fuels lesion proliferation and inflammation.
This study elucidates the characteristics of ectopic endometrial cells and their interactions within the lesion microenvironment, providing crucial insights into endometriosis pathogenesis and highlights WNT5A as a promising therapeutic target.
Professor Leng emphasized ongoing efforts to translate these findings into precision therapies that "target pathological cells without harming healthy tissues." . Academician Lang Jinghe underscored PUMCH’s commitment to advancing multidisciplinary research to improve the lives of endometriosis patients.
Written by Chen Xiao
Pictures courtesy of Liu Song
Edited by Xiao Xiong
