Recently the prestigious international journal Circulation (IF: 35.5) published the findings of a collaborative research involving teams led by Professor Zhang Shuyang, President of PUMCH, and Associate Researcher Hu Xiaomin, along with Researcher Zhang Yan's team from the Institute of Cardiovascular Sciences, School of Basic Medical Sciences, Peking University; Academician Dong Erdan's team from Peking University Third Hospital; and Associate Researcher Li Ting's team from the First Affiliated Hospital of Xi'an Jiaotong University. The research has provided important insights into the pathogenesis, prognosis prediction, and therapeutic and prevention strategies for cardiac ischemia/reperfusion (I/R) injury. Professor Richard N. Kitsis, Director of the Wilf Family Cardiovascular Research Institute at Albert Einstein College of Medicine, wrote an accompanying editorial for this publication.
A persistent clinical challenge in disease treatment is the occurrence of new injuries during therapeutic interventions, such as cardiac ischemia/reperfusion (I/R) injury following reperfusion therapy including percutaneous coronary intervention (PCI). With 30-40% of acute myocardial infarction (AMI) patients experiencing major adverse cardiovascular events (MACEs) within five years after PCI, identifying effective biomarkers for early risk detection and precise intervention is crucial.
Receptor-interacting protein kinase 3 (RIPK3) plays a vital role in regulating inflammatory signals and cell death pathways. Early research by Zhang Shuyang and Hu Xiaomin’s team found that RIPK3 can be detected in peripheral blood and serves as a biomarker for various cardiovascular diseases, though it’s unclear whether it has biological functions in extracellular environment.
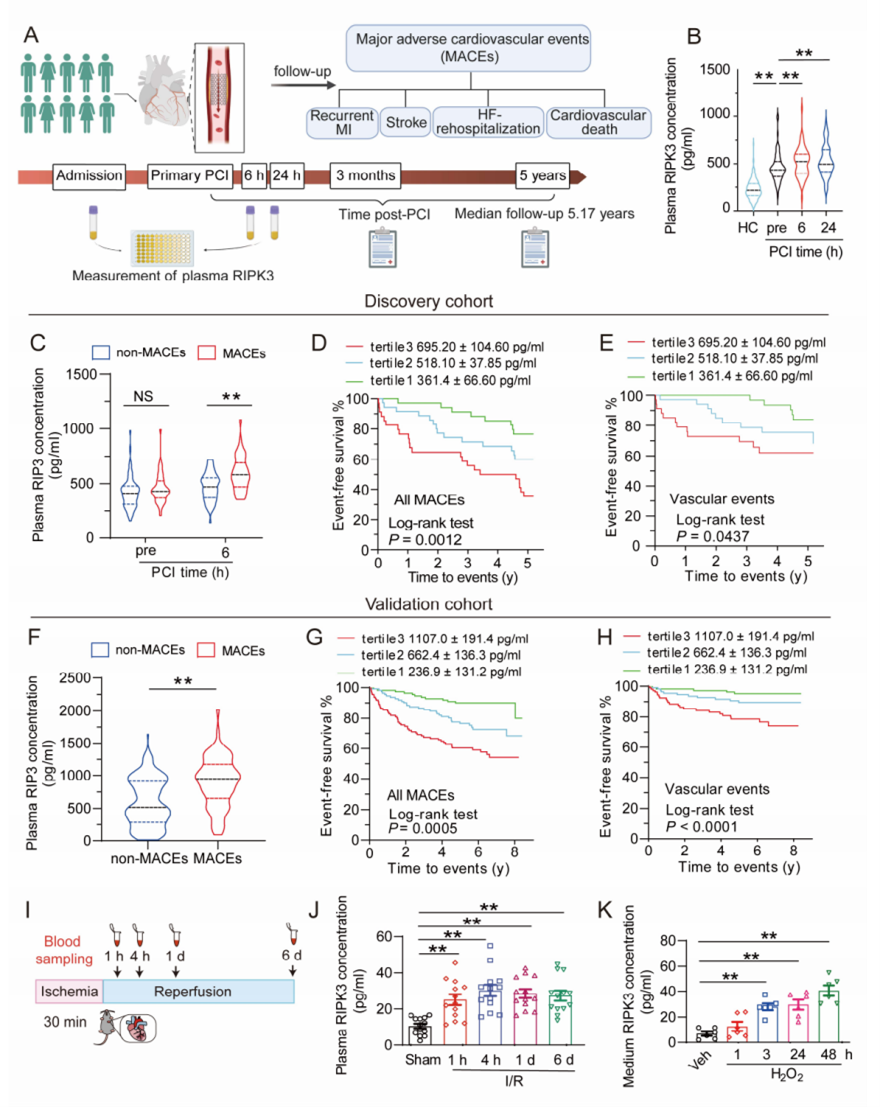
▲Positive correlation between extracellular RIPK3 concentrations and MACEs risk in AMI patients receiving PCI
The study enrolled 437 AMI patients receiving PCI and found significantly higher plasma RIPK3 levels at 6 hours post-PCI in the MACEs group compared to the non-MACEs group. Through animal experiments, the team discovered that extracellular RIPK3 affects cardiomyocytes, inflammatory cells, and endothelial cells, causing injury, inflammation, and dysfunction in these cells, thereby exacerbating cardiac I/R-induced inflammation and cardiovascular injury; blocking extracellular RIPK3 with neutralizing antibodies improved cardiac I/R injury in mice. Mechanistically, RIPK3 acted as a damage-associated molecular pattern and bound with RAGE (receptor of advanced glycation end-products), subsequently activating CaMKII (Ca2+/calmodulin-dependent kinase II) to elicit the detrimental effects in cardiomyocytes, macrophages, and endothelial cells.
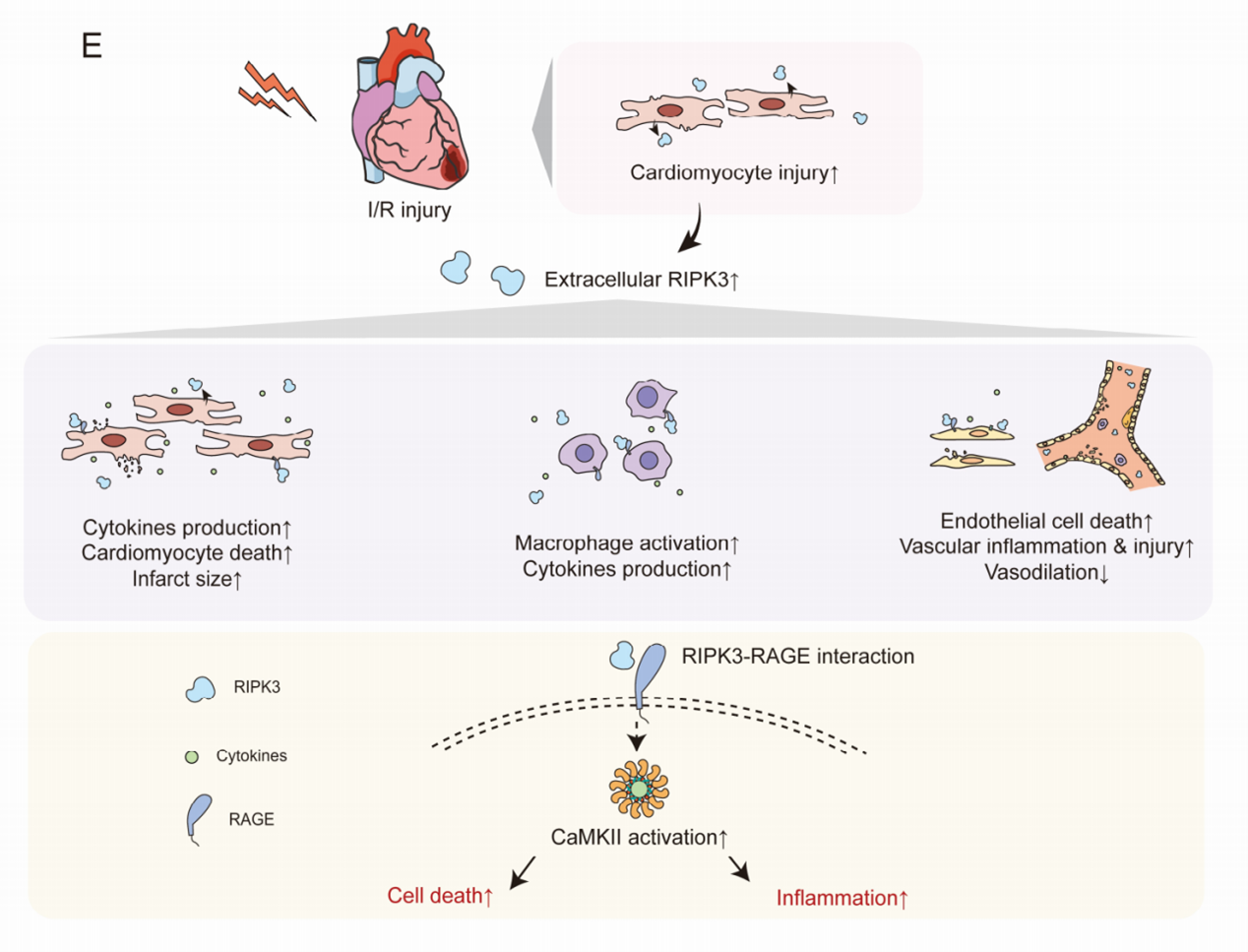
▲Mechanism diagram showing extracellular RIPK3 exacerbating cardiac I/R injury through RAGE-CaMKII pathway
These findings demonstrate that plasma RIPK3 levels positively correlate with MACEs occurrence in AMI patients undergoing PCI, making RIPK3 an important biomarker for post-PCI MACEs risk stratification. Extracellular RIPK3's involvement in mediating cardiac I/R injury suggests potential new strategies for preventing and treating cardiac I/R injury and its complications. This discovery has significant implications for AMI patient risk stratification and treatment, potentially improving patient outcomes and reducing cardiovascular events related to cardiac I/R injury.
In his accompanying editorial titled “Extracellular Role for the Intracellular Cell Death Mediator RIPK3 in Myocardial Infarction”, Professor Richard N. Kitsis strongly acknowledged the significance of these findings and anticipated their validation in larger patient populations.
Written by Chen Xiao
Pictures courtesy of the Department of Cardiology
Edited by Xiao Xiong
