Recently, Professor Zhao Chan's ophthalmology team at PUMCH published three research breakthroughs in prestigious journals that are ranked among the top 5% by the Chinese Academy of Sciences. The first study, published in the American Journal of Ophthalmology, introduces a novel risk-stratified treatment strategy for Vogt-Koyanagi-Harada disease (VKH). This approach has the potential to enhance treatment precision for VKH patients, helping avoid both overtreatment and undertreatment. The other two original studies, focusing on innovative intraocular drug delivery systems, were published in Advanced Science and Chemical Engineering Journal respectively. These findings offer promising new strategies for early intervention in diabetic retinopathy and age-related cataract respectively.
1. Presenting the Pioneering Risk-stratified Treatment Strategy for Vogt-Koyanagi-Harada Disease to the International Community
Vogt-Koyanagi-Harada disease (VKH) is one of the most common types of uveitis among the Chinese population. Currently, there is no globally uniform treatment protocol. The traditional approach involves high-dose glucocorticoids, with immunomodulatory therapy (IMT) agents added as-needed when glucocorticoids prove ineffective, intolerable, or in cases of relapse. Some researchers argue for the immediate use of immunomodulatory therapy (IMT) agents for VKH patients to reduce the probability of recurrence.
After years of clinical observation, Professor Zhao Chan and Professor Zhang Meifen's team discovered significant individual differences in VKH patients' responses to glucocorticoid treatment and prognosis. They were the first to report internationally several imaging features significantly correlated with the prognosis, including choroidal folds and subretinal exudation/fibrosis. Building on this and previous research on prognostic factors, they pioneered criteria for stratifying VKH patients into high-risk and low-risk groups, who received different treatment regimens of different intensity. Through clinical observation, the team confirmed the scientific validity of their stratification criteria and the stratified treatment regimens.
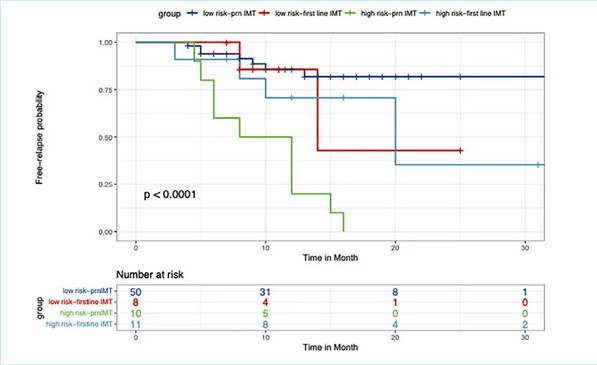
▲Kaplan-Meier survival analysis of VKH recurrence in high-risk and low-risk groups with two IMT regimens
2. Intravitreal Injection of Glucose-Responsive Hydrogel Inhibits Blood-Retinal Barrier Injury
Local high oxidative stress levels in the retina, abnormal activation of immune cells, and blood-retinal barrier (BRB) injury are crucial mechanisms in causing early diabetic retinopathy (DR). These mechanisms mutually reinforce each other, collectively forming a complex retinal microenvironment (RME) that causes sustained damage to neurovascular units. The research teams led by Professor Li Nan from the School of Pharmaceutical Science and Technology at Tianjin University and Professor Zhao Chan jointly developed a glucose-responsive hydrogel named Cu-PEI/siMyD88@GEMA-Con A (CSGC), which effectively delivers Cu-PEI nanoparticles (NPs) and siMyD88 (which restores normal expression of tight junctions) to the retinal pigment epithelium (RPE) cells in experimental animals.
Cu-PEI NPs efficiently scavenge reactive oxygen species (ROS) in the RME, thereby effectively inhibiting primary BRB injury and the resulting immune cascade reactions. The action of siMyD88 synergizes with Cu-PEI NPs, promoting the expression of various junction complexes in the RME, thus blocking secondary BRB injury.
This study provides a new strategy for treating diabetic retinopathy via remodeling the retinal microenvironment, which has significant research implications and potential for clinical application.
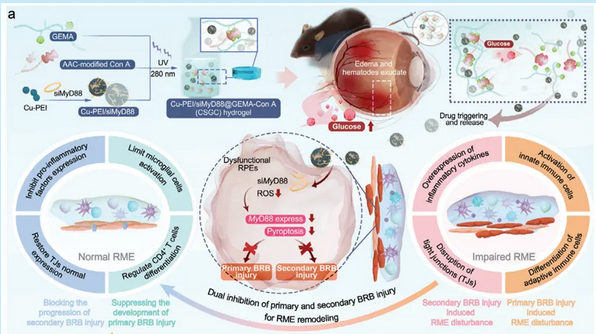
▲Schematic illustration showing that the CSGC hydrogel inhibits primary and secondary BRB injury to promote retinal microenvironment remodeling in diabetic retinopathy
3. Development of a Novel Bilirubin Nanoparticle Eyedrop, Effectively Inhibiting Ultraviolet-Induced Cataract
Age-related cataract (ARC) is the leading cause of blindness worldwide, currently lacking effective medications to slow disease progression. Building on previous research demonstrating that bilirubin (BR) at physiological concentrations can protect lens epithelial cells (LECs) from oxidative stress damage and cellular senescence, Professor Zhao Chan's team, in collaboration with Professor Qian Feng's team from the School of Pharmaceutical Sciences at Tsinghua University and the team from Tongji University School of Medicine in Shanghai, has pioneered the use of PEG-PLGA (polyethylene glycol-poly(lactic-co-glycolic acid)) nanoparticles to encapsulate BR. These nanoparticles were further modified with cRGD to create cRNM@BR eyedrops, enhancing BR's solubility and transcorneal penetration. The study investigated the efficacy and mechanism of this nanodrug in delaying ultraviolet B (UVB)-induced cataract in mice.
The research found that cRNM@BR eyedrops increased the drug's retention time on the ocular surface and achieved efficient transcorneal transport through the ER/Golgi pathway, significantly elevating BR concentration in the anterior chamber. Histopathological studies confirmed that these eyedrops could delay the progression and mitigate the severity of UVB-induced cataract in mice by reducing oxidative stress in lens cells, restoring activity of DNA repair enzymes, and inhibiting lens epithelial cell senescence. Transcriptomics analysis revealed for the first time that cRNM@BR could inhibit lens epithelial cell senescence and lens opacity by regulating the dsDNA/cGAS/STING pathway. This study offers a promising new strategy for early intervention in age-related cataract, with potential for translational application.
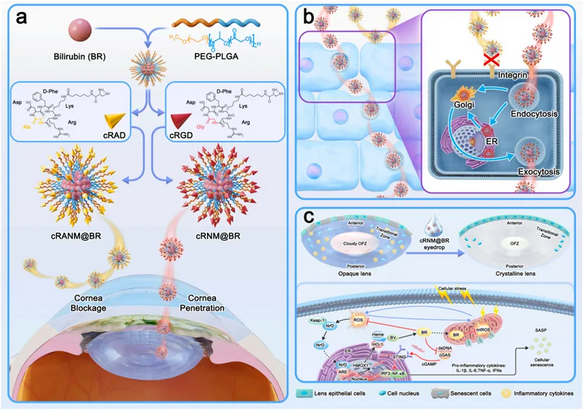
▲Schematic illustration of the preparation of cRNM@BR for preventing against UVB-induced cataract
Written by Zhao Chan
Reviewed by Chen Youxin and Zhang Meifen
Pictures courtesy of the Department of Ophthalmology
Edited by Wang Jingxia
