Recently, a team led by Dr. Yang Huaxia, Deputy Chief Physician of the Department of Rheumatology, and Dr. Yang Bo, Deputy Director of the Department of Orthopedics at PUMCH, published their original research in “Nature Communications” (IF: 16.6), revealing a novel immunological mechanism underlying PD-1 inhibitor-induced inflammatory arthritis (PD-1-IA). The team discovered distinct immune cell and molecular characteristics of PD-1-IA that are different from traditional rheumatoid arthritis (RA), highlighting IL1Bhi macrophages as a possible therapeutic target in PD-1-IA. This research was supported by the National High Level Hospital Clinical Research Funding.

Immune-related inflammatory arthritis (IA) is one of the most common rheumatic adverse events following immune checkpoint inhibitors treatment. Unlike common forms of arthritis such as RA, IA patients often lack diagnostic biomarkers in their peripheral blood that can aid in diagnosis and treatment. This study focused on addressing this clinical challenge of IA by elucidating its immunopathogenic mechanisms.
The study enrolled patients who developed IA after receiving anti-PD-1 monoclonal antibody treatment and collected synovial fluid and peripheral blood immune cells from them. Combining single-cell transcriptomic sequencing and verification through functional experiments, the researchers compared the unique immune cell subpopulations and functions in IA patients with those of RA patients and of healthy individuals.
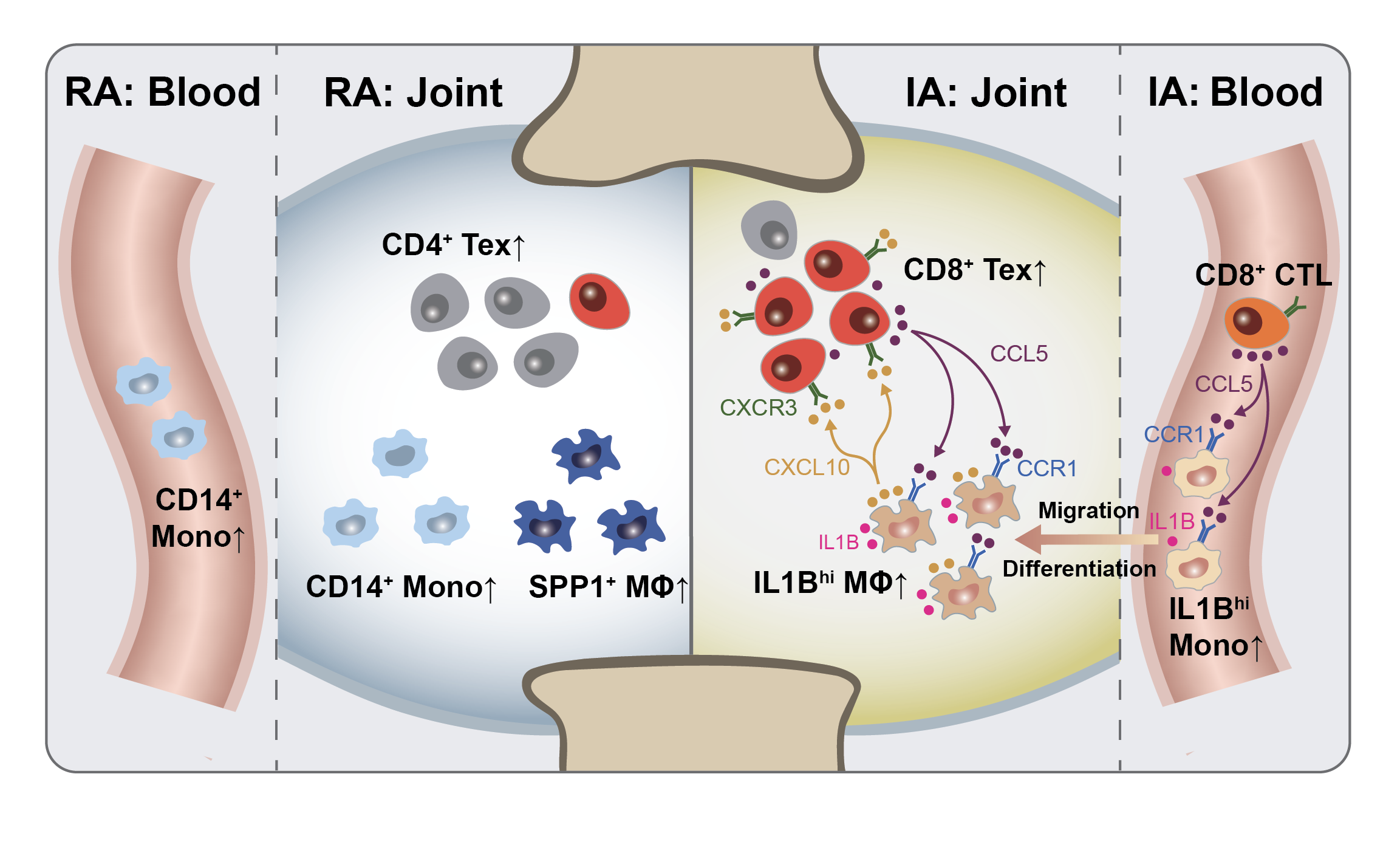
▲Graphical summary of this study
For the first time, the study identified a unique pro-inflammatory myeloid cell subcluster in PD-1-IA patients, which were abundant in the synovial fluid and peripheral blood of these patients but rarely found in healthy individuals or RA patients. These cells had high gene expression of IL1B and upregulated genes related to inflammatory pathways such as TNFα and NLRP3 inflammasome, exhibiting pro-inflammatory functions. Gene expression analysis suggested that the myeloid cells in the joint cavity were likely from the peripheral blood. The team also identified a highly proliferative, exhausted CD8+ T subcluster in the synovial fluid, contributing to the sustained inflammatory response in IA. Through cell-cell communication analysis, the researchers discovered that the pro-inflammatory IL1Bhi macrophages closely communicated with the exhausted CD8+ T cells via the CCR1-CCL5/CCL3 and CXCL10-CXCR3 ligand-receptor pairs, setting in motion a “vicious cycle” that promoted inflammation and drove the development of IA. This mechanism differs significantly from the pathogenesis of traditional RA.
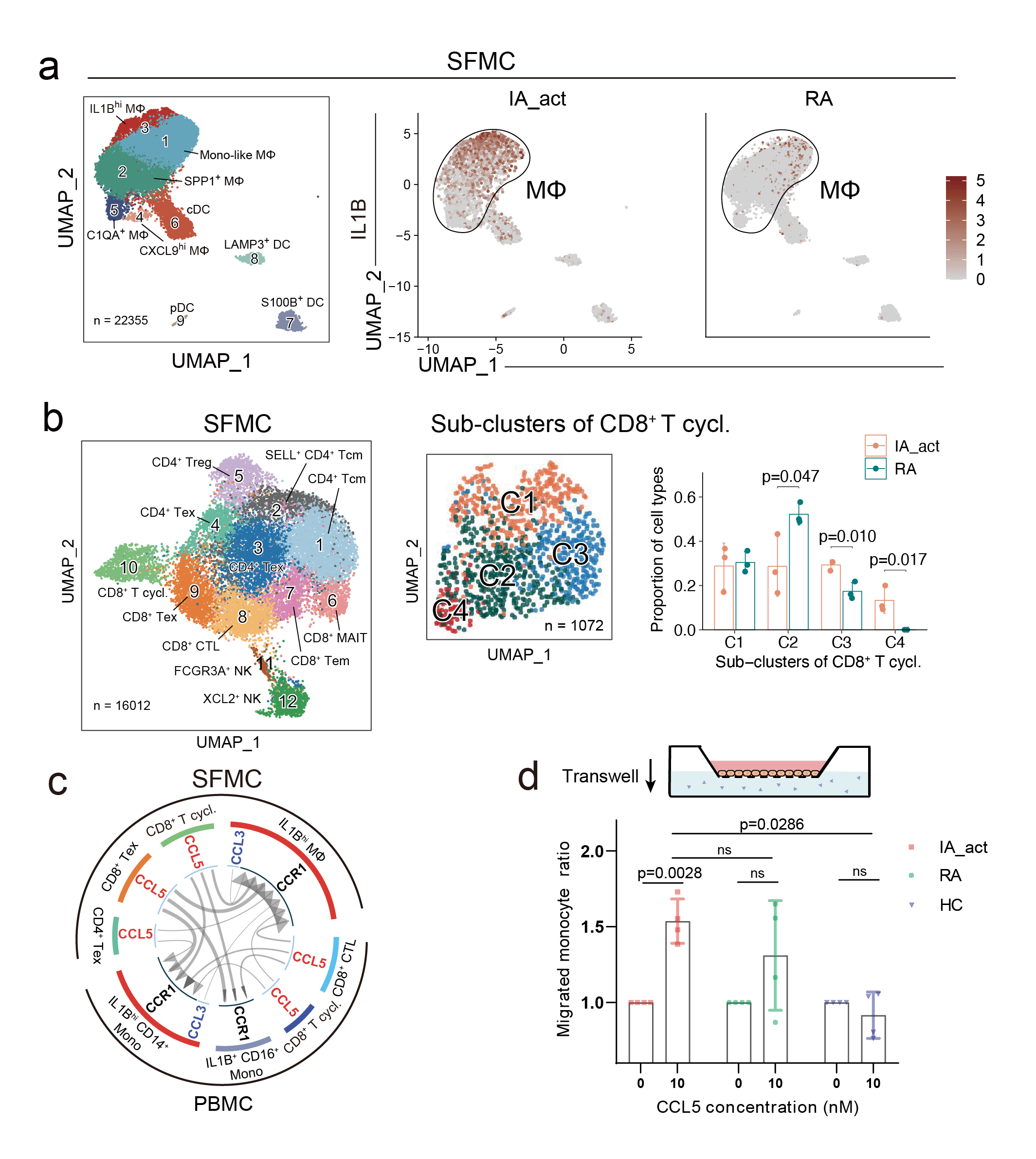
▲The pathogenesis mechanism
This study elucidated the immunopathogenic mechanism of IA that is different from RA at the single-cell level, providing a novel perspective for understanding and potential therapeutic targets for treating rheumatic immune-related adverse events. Currently, research on the mechanisms of adverse events caused by PD-1 inhibitors has primarily focused on T cell dysfunction. This study is the first to discover the crucial pathogenic role of pro-inflammatory myeloid cells in PD-1-IA, shedding light on the connection between the abnormalities in innate and adaptive immunity, and thus providing a more comprehensive understanding of the pathological mechanisms underlying PD-1-IA.
Written by Zhou Ziyue, Jiang Xu and Yang Huaxia
Edited by Chen Xiao and Gan Dingzhu
Translated by Liu Haiyan
Reviewed by Jiang Nan and Wang Yao
