Recently, the team led by Zhang Fengchun from the Department of Rheumatology at PUMCH published an article online in the top international journal “Annals of the Rheumatic Diseases” (IF: 27.4), which reported the results of a phase 2b, randomized, double-blind, placebo-controlled trial. The study provided preliminary evidence of the effectiveness and safety of Telitacicept, a novel biologic agent developed by China independently, in treating active systemic lupus erythematosus (SLE). It offers a new treatment option for patients with inadequate response to conventional therapies and also establishes a solid foundation for future global multicenter Phase III studies.

Currently, many SLE patients don’t benefit much from treatment, suffering decreased quality of life, target organ damage, comorbidities, and even premature death. B cells play a central role in the development of SLE, and BLyS (B lymphocyte stimulator) and APRIL (a proliferation-inducing ligand) are two key factors that regulate B cell differentiation, survival, maturation, and antibody secretion. Telitacicept is a novel biologic agent developed in China. When injected into patients, it can simultaneously antagonize the biological activity of both BLyS and APRIL, thereby enabling better control of SLE.
This national multicenter clinical trial led by Zhang Fengchun recruited from 29 medical centers in China 249 patients aged between 18 and 65 with active SLE, who had moderate to high disease activity despite receiving conventional treatment. These patients were randomized 1:1:1:1 to receive subcutaneous Telitacicept at 80 mg, 160 mg, 240 mg or placebo once weekly.
The results showed that at week 48, all three Telitacicept treatment groups demonstrated significant superiority over the placebo group (Figure A). The 160mg dose group of Telitacicept (the approved standard dose in China) exhibited a significant advantage over the placebo group starting from week 12 and maintained this advantage until week 48 (Figure B). Safety analysis indicated that Telitacicept was well-tolerated, with a similar incidence of adverse events and serious adverse events to the placebo. However, local reactions at the injection site were more common in the Telitacicept groups than in the placebo group.
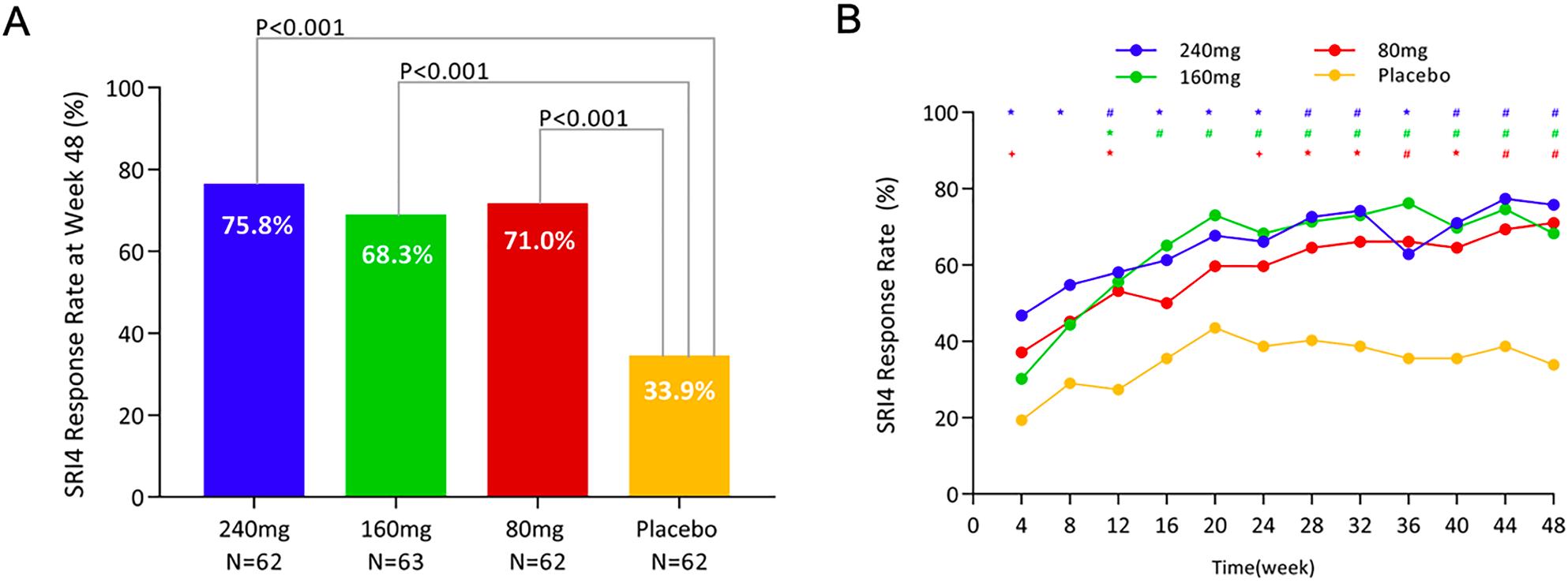
This study has contributed to the conditional approval of Telitacicept, a novel original drug, in China. It represents a positive contribution from the Chinese medical community to SLE patients worldwide. Additionally, it has provided encouraging and solid data support for ongoing larger-scale global multicenter Phase III clinical trials.
Translated by Liu Haiyan
Reviewed by Jiang Nan and Wang Yao
