On December 13, 2023, the research results of the Chinese clinical study named “WU-KONG6”, led by Professor Wang Mengzhao from the Department of Pulmonary and Critical Care Medicine, PUMCH, were published as an entire article in the internationally renowned “The Lancet Respiratory Medicine” (IF: 76.2). The study focused on the use of Sunvozertinib, a Chinese Class I innovative drug, for the treatment of advanced non-small cell lung cancer (NSCLC) patients with EGFR exon20 insertion mutation (EGFR exon20ins) who progress after or are intolerant to platinum-based chemotherapy. The study demonstrated significant anti-tumor efficacy of Sunvozertinib and exhibited good safety, showcasing its “best-in-class” potential for NSCLC patients with EGFR exon20ins mutation. This research finding has made a huge difference for such patients in China as a standard treatment option with high efficacy and low toxicity. Based on the results of this study, Sunvozertinib obtained regulatory approval and was launched in China in August 2023. It is the first and only Class I innovative drug for NSCLC with EGFR exon20ins mutation.

Lung cancer is the most common malignant tumor in China, with the highest incidence and mortality rate. Approximately 50.2% of NSCLC patients with types of adenocarcinoma have EGFR gene mutations. EGFR exon20ins mutations, as primary mutations, account for about 12% of all EGFR mutations. Due to the large population of NSCLC patients in China, individuals with EGFR exon20ins mutations are large in number and merit serious attention. However, safe and effective targeted treatment options for such mutations have been lacking due to their unique spatial configuration and high heterogeneity. Patients with EGFR exon20ins mutations have had limited survival benefits and are in dire need of better treatment options.
To address this clinical challenge and increase patients’ survival benefits, Professor Wang Mengzhao from the Department of Pulmonary and Critical Care Medicine at PUMCH led a Chinese clinical study “WU-KONG6” on Sunvozertinib, a Class I innovative drug in China. The study aimed to evaluate the efficacy and safety of Sunvozertinib in the treatment of advanced NSCLC patients with pretreated EGFR exon20ins mutations. With the strong support of the Clinical Pharmacology Center at PUMCH and the collaborative efforts of 37 medical centers nationwide, the Phase II clinical trial WU-KONG6 was well executed and successfully completed.
A total of 104 advanced NSCLC patients with EGFR exon20ins mutations who progressed after or were intolerant to chemotherapy were enrolled in the study. They were treated with oral monotherapy of Sunvozertinib at a dosage of 300mg per day. According to research results: among the 97 patients evaluable for efficacy analysis, ORR of the Sunvozertinib monotherapy was 61%, over 90% of patients achieved tumor shrinkage in target lesions, and the ORR was consistently above 50% across different subtypes of EGFR exon20ins mutations. Furthermore, Sunvozertinib is well-tolerated and has a similar safety profile to EGFR-TKIs, and it is clinically manageable and reversible.
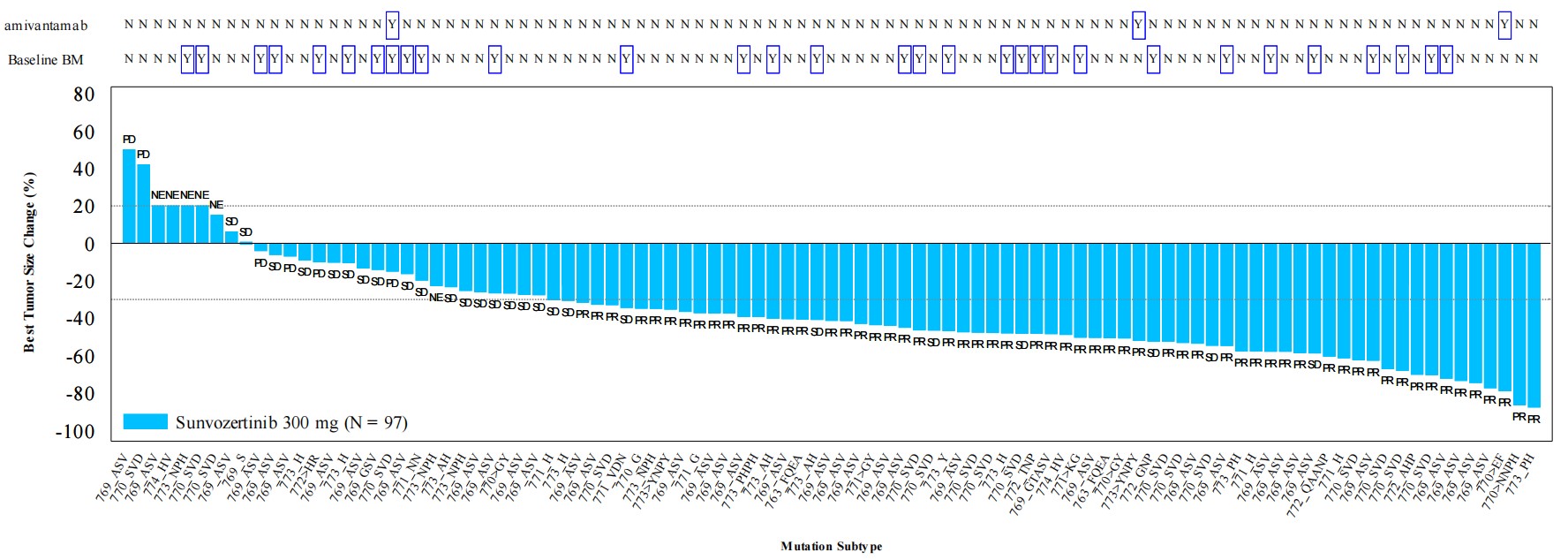
▲Tumor size change evaluated by IRC (independent review committee)
In addition, three other studies are underway: WU-KONG15, a clinical study of the same series, led by Prof. Wang Mengzhao; the international multicenter studies WU-KONG1 Part B and Wu-Kong28, in which Prof. Wang Mengzhao’s team plays a major role.
Over the years, the Department of Pulmonary and Critical Care Medicine at PUMCH has been making continuous efforts in the diagnosis and treatment of lung cancer patients. Be it systemic chemotherapy, small-molecule targeted therapy or immunotherapy, PUMCH teams have always been at the forefront of clinical research, continuously improving diagnostic and treatment techniques, and actively exploring and leading the way for Chinese original drugs and innovative drugs to the international stage. This has effectively improved the survival time and quality of life for a larger number of patients.
Written by Xu Yan and Yan Xiaobo
Pictures courtesy of the Department of Pulmonary and Critical Care Medicine
Translated by Liu Haiyan
Reviewed by Tian Xinping and Wang Yao
