Recently, the “Guideline for the Application of Chromosomal Microarray Analysis in Prenatal Diagnosis (2023)” was published in both the “Chinese Journal of Obstetrics and Gynecology” and the “Chinese Journal of Medical Genetics”. The guideline was developed by the Department of Obstetrics and Gynecology of PUMCH as the Secretary General unit of the National Expert Group on Prenatal Diagnosis of the Department of Maternal and Child Health under the National Health Commission, in collaboration with eight prenatal diagnosis institutions nationwide. Supported by the National High Level Hospital Clinical Research Funding, this guideline helps promote the standardized application of chromosomal microarray analysis (CMA) in prenatal diagnosis in China.


Copy number variations (CNVs) in the genome, including deletions and duplications of chromosome segments, are major causes of genetic diseases and birth defects. CMA can simultaneously detect almost all microscopic and submicroscopic CNVs in the genome, significantly improving the resolution of karyotype analysis. In 2014, Professor Bian Xuming from the Department of Obstetrics and Gynecology at PUMCH led the National Expert Group on Prenatal Diagnosis to develop the first expert consensus on CMA in prenatal diagnosis in China, providing strong support for the promotion and application of CMA technology in the country.
After more than eight years of development, CMA has become a first-line prenatal diagnostic technique for detecting CNVs involving chromosomal deletions or duplications in fetuses. It is now widely used in prenatal diagnosis. With the evolution of the technology and the accumulation of the diagnosed cases, further standardization of the technology is urgently needed. In 2020, with the support of the Chinese Preventive Medicine Association and the relevant academic groups under the Chinese Society of Medical Genetics, the Prenatal Diagnosis Center of PUMCH took the lead in initiating the formulation of the CMA application guideline. Based on discussions and feedback, as well as extensive correspondence reviews and revisions from experts on prenatal diagnosis nationwide, the CMA application guideline was officially published in August 2023.
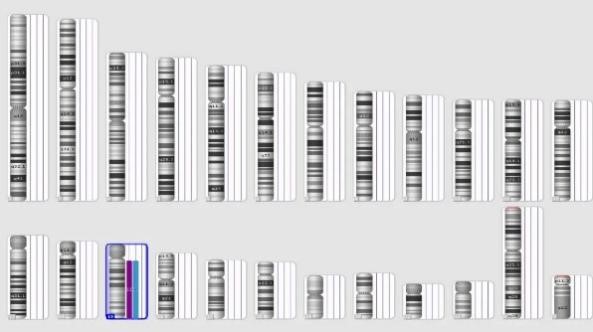
▲Left: Simulated karyogram of the complete set of chromosomes. Right: Diagram of mosaic duplications and loss of heterozygosity of chromosomes
The CMA guideline (2023) consists of six chapters, providing clear views and recommendations on important issues such as clinical indications, laboratory testing and quality control, pathogenicity interpretation, report format and content, pre- and post-test clinical counseling, result validation and extended testing in special circumstances, and so on. This guideline is a comprehensive aggregation of the experience, thoughts, and guidance of experts on prenatal diagnosis nationwide and thus can be used as a powerful tool. The guideline proposes eight clinical indications for prenatal diagnosis, including five new ones, and provides recommendation classification for each indication. According to the guideline, quality control is a necessary step for all five laboratory operation procedures, with particular emphasis on confirmation of the fetal origin of the tested samples before testing. The guideline recommends reporting only three types of CNVs: pathogenic, likely pathogenic, and variants of uncertain significance, and does not recommend reporting CNVs classified as likely benign and benign according to the American College of Medical Genetics and Genomics guidelines (ACMG, 2019).

▲A CMA testing is being conducted in the laboratory of the Prenatal Diagnosis Center of PUMCH.
Prof. Liu Juntao from the Department of Obstetrics and Gynecology emphasized the importance of comprehensive and objective pre- and post-test genetic counseling. Before the test, clinical physicians should strictly adhere to the application indications. For pregnant women who meet the testing criteria, the pregnant women and their families should be fully informed about the applicable scope of the technology, as well as the testing range, accuracy, limitation, turnaround time and cost, etc, so that they can make an fully informed decision and sign the consent form. After testing, CMA results should be interpreted integrated with clinical information of both the fetus and the pedigree.
The Department of Obstetrics and Gynecology at PUMCH is actively organizing training sessions to help obstetric and gynecological colleagues across the country better understand and apply the CMA guideline. Over 2,600 participants from over 500 prenatal diagnosis centers nationwide signed up for the online guideline interpretation meeting held on the 19th China Birth Defects Day and had animated exchanges about topics covered.
If you are interested, please read the paper on the webpage https://rs.yiigle.com/cmaid/1470870.
Written by Hao Na, Li Mengmeng and Fu Tanpin
Edited by Xiao Xiong
Translated by Liu Haiyan
Reviewed by Lyu Yan and Wang Yao
