On January 17, 2023, the extracorporeal membrane oxygenation (ECMO) device “Huisheng-I”, jointly developed by PUMCH and China Aerospace Science and Technology Corporation, among others, was approved by the National Medical Products Administration (NMPA) for registration. The device is a groundbreaking achievement of applying China’s aerospace technology in the medical field. It is a fully China-developed extracorporeal cardiopulmonary support system with completely independent and controllable key technologies, core components and materials. The device is a case in point about how to effectively remove bottlenecks in the R&D of domestic high-end medical devices.
The domestic ECMO approved for registration is the outcome of a highly integrated and optimized design of the whole ECMO system by the R&D team that strove to satisfy the clinical needs and avoid flaws of existing products and ensured that key technologies, core components and materials were all completely independently developed and controllable. At present, the device has passed the bench test, reliability test and animal test, which have preliminarily validated the safety and efficacy of the system and its adaptability with the supporting consumables. The interim analysis report of the clinical trial has been approved by NMPA, hence the approval for marketing.
As early as the beginning of 2020, PUMCH joined this interdisciplinary and medical-industrial ECMO research taskforce, mainly tasked with the design and implementation of animal experiments and clinical trials. Professor Du Bin, then Director of Medical ICU of PUMCH, served as the Deputy Chief Commander of the domestic ECMO initiative.
At present, the COVID control has entered a new phase in China where the goal has become “ensuring health and preventing critical illness”. To provide better care to patients in critical conditions, it is necessary to coordinate and optimize medical resources allocation. The approval of the domestic ECMO device will, to a certain extent, ease the urgent clinical demand for ECMO. Besides, as a key project supported for high-level hospital development, it will also be a case in point for subsequent medical-industrial integration projects.
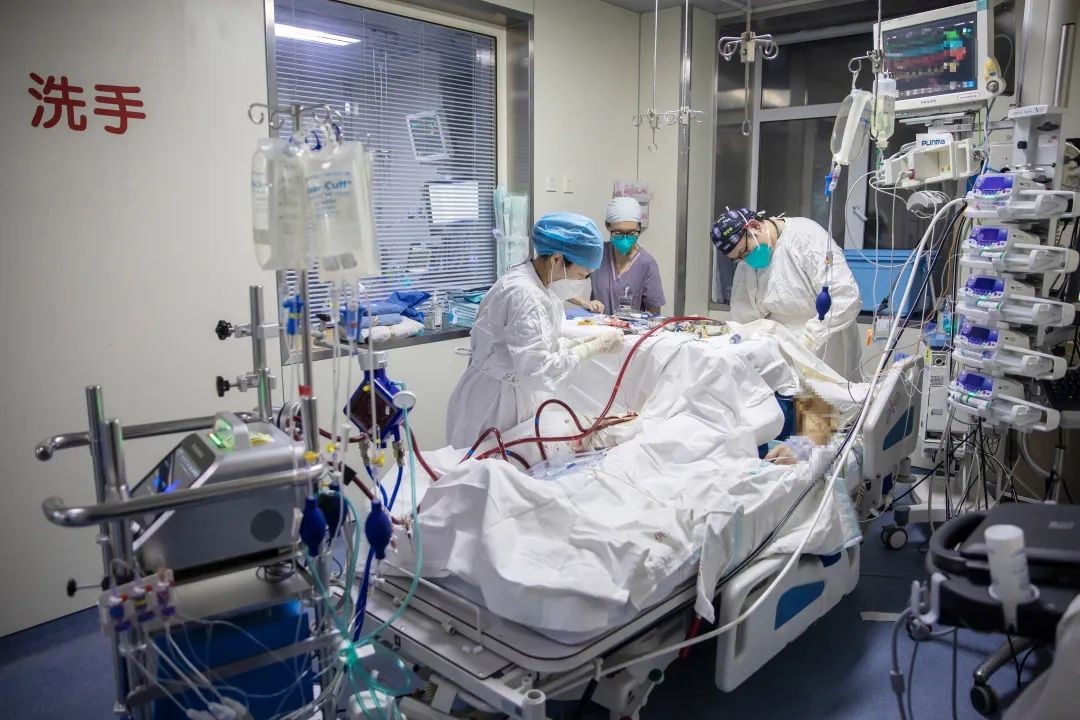
Doctors and nurses of Medical ICU were using the domestic ECMO to save patients (in the clinical trial phase)
Written by: Chen Xiao and Wang Chunyao
Photographer: Sun Liang
Translator: Liu Haiyan
Editor: Wang Yao
