
Professor Zhi-Cheng Jing from the department of cardiology, Peking Union Medical College hospital discovered a novel susceptibility gene PTGIS for idiopathic pulmonary hypertension (IPAH). PTGIS gene encodes prostacyclin synthase (PTGIS) the mutation of which could explain the etiology of 6.1% in IPAH patients. The study also found that patients carrying rare variants of PTGIS were more responsive to prostacyclin treatment than those without such variants, and may provide guidance on tailored therapies for managing these patients. The findings were published online April 1 in JAMA Cardiology, a top cardiovascular journal.

IPAH is a rare disease with poor prognosis. The pathogenesis is complicated and largely unknown. BMPR2 is the most predominant causal gene of IPAH, however its variants only account for 14.5% of IPAH patients in China. In a substantial portion of IAPH cases, disease-associated genes have not been identified.
Professor Zhi-Cheng Jing and his team engaged in clinical and basic research of pulmonary hypertension for two decades. In 2019, professor Jing’s team completed the first genome-wide genetic study on the large sample of IPAH clinical cohort in China and discovered the new IPAH pathogenic gene BMP9, which attracted great attention of the international academic community. Therefore, the leading cardiovascular journal, European Heart Journal, published a full-length report to introduce the remarkable achievements of professor Jing's team in genetics of cardiovascular diseases, cohort studies and the development of new drugs at the end of 2019. Another breakthrough came recently when the team discovered another IPAH associated gene: prostacyclin synthase (PTGIS). The paper was published in JAMA Cardiology, a top cardiovascular journal.

At first, Jing's team sequenced the entire genome of 42 IPAH patients without BMPR2 mutations and identified the enrichment of rare variants in the PTGIS gene. Subsequently, the validation of the expanded sample showed that the occurrence frequency of rare mutations in PTGIS gene in IPAH patients was 6.1% (14/230), which is significantly higher than that of 0.8% (8/968) in healthy people which increased 7.8 higher odds of pulmonary arterial hypertension (95%CI, 3.2~18.8; P=5.0×10-6).
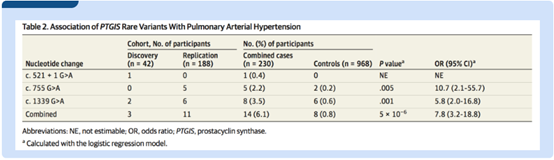
Three rare mutations of PTGIS gene were found in IPAH patients: one spliced site mutation (c.521+1G>A) and two missense mutations (p.R252Q and p.A447T). Functional studies have shown that all of these three variations lead to PTGIS protein loss-of-function: the splicing site variation leads to incorrect mRNA splicing and two missense mutations significantly reduced the ability of PTGIS protein to synthesize prostaglandin (PGI2), and also reduced the endothelial cell–protective function of PTGIS protein, making endothelial cells more prone to apoptosis and damage.
More exciting finding is the strong correlation between rare PTGIS variants and pulmonary vascular reactivity in IPAH patients. The linear regression model study showed that, compared with patients without PTGIS variation, the pulmonary vascular resistance of patients with rare variation decreased more significantly (95%CI, -3.40~-0.87; P=0.001) and the cardiac function index increased more significantly after Iloprost inhalation (95%CI, 0.19~0.65; P < 0.001). This suggested that genetic variants of PTGIS predisposed pulmonary vascular responses to the iloprost stimulation, thereby providing a rationale for targeting the prostacyclin pathway as part of tailored therapies for managing patients with PTGIS variants, and indicating its important clinical value.
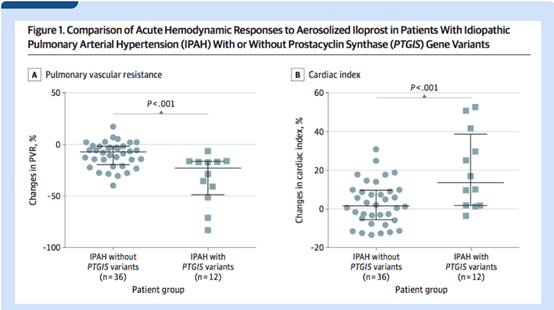
Acute hemodynamics, including pulmonary vascular resistance and cardiac index, were significantly different in patients with pulmonary hypertension with PTGIS gene mutation and those without PTGIS gene mutation after Iloprost inhalation, suggesting that PTGIS gene could be an important target for future pharmacogenomics research.
The discovery of rare mutations in the PTGIS gene is a dramatic breakthrough, not only explaining the etiology of the additional 6.1% IPAH patients, but also helping improve personalized treatment of these patients.
Professor Jing said he believed that pulmonary arterial hypertension has left a cipher, leading to the cure of the disease, since it appeared. And researchers' mission was to decode the cipher to find the cure of IPAH. Under the guidance of professor Jing, his team once again cracked the genetic code of IPAH after discovering BMP9, the second pathogenic gene among Chinese IPAH patients in 2019.
Associate professor Xiao-Jian Wang, associate professor Xi-Qi-Xu and associate professor Kai Sun were the co-first authors of the paper. Professor Zhi-Cheng Jing and professor Xue Zhang were the co-corresponding authors. Professor Shu-Yang Zhang was fully involved in the design and implementation of the study. The research was funded by the Beijing Natural Science Foundation, the National Key Research and Development Program of China, the National Key Research and Development Program of China and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences.
https://doi.org/10.1001/jamacardio.2020.0479
Writer: Xian-Mei Li, Si-Jin Zhang, Hui Wang
Photo: Department of Cardiology, PUMCH
