Recently, an original research article by the team of Chief Physician Zeng Xiaofeng, Director Li Mengtao, and Deputy Director Zhao Jiuliang of the Department of Rheumatology at PUMCH, in collaboration with Professor Du Guanhua's team from the Institute of Materia Medica, Chinese Academy of Medical Sciences, was published online in The Innovation (IF: 25.7). For the first time, this study identified through large-sample genetic analysis that rare coding variants in the TRAF5 gene are significantly enriched in patients with systemic lupus erythematosus-associated pulmonary arterial hypertension (SLE-PAH). It further revealed the key mechanism by which these variants promote the occurrence and progression of PAH by causing endothelial cell dysfunction, providing new insights for early warning and treatment of this condition.

Pulmonary arterial hypertension (PAH) is one of the most severe complications of systemic lupus erythematosus (SLE), characterized by an insidious onset and a very poor prognosis. This study, based on the prospective cohort of the Chinese SLE Treatment and Research Group (CSTAR), conducted genetic research involving 241 SLE-PAH patients, 736 SLE patients without PAH, and 996 healthy controls through whole-exome sequencing analysis. Gene-based burden analysis revealed that rare deleterious variants in the TRAF5 gene were significantly enriched in SLE-PAH patients (3.78%), markedly higher than in the SLE-nonPAH group (1.37%) and the healthy control group (1.13%). These variants included splice-site mutations, frameshift mutations, and missense mutations. In vitro experiments confirmed that these variants lead to decreased expression or loss of function of the TRAF5 protein.
To elucidate the role of TRAF5 in disease pathogenesis, the team generated TRAF5 gene knockout mice and established an SLE-PAH model using a dual-hit SLE-PAH model with pristane and hypoxia (PtHx) induction. The results showed that TRAF5 knockout mice had significantly reduced survival rates during the modeling process and exhibited aggravated typical PAH pathological changes. Single-cell RNA sequencing of lung tissue further revealed that TRAF5 deficiency led to upregulation of apoptosis-related genes and downregulation of BMP signaling activity in arterial endothelial cells. At the cellular level, knocking down TRAF5 in pulmonary arterial endothelial cells resulted in impaired cell function and disruption of the BMP/TGF-β signaling balance. Conversely, endothelium-specific overexpression of TRAF5 attenuated the pulmonary hypertension phenotype in model mice, demonstrating its potential therapeutic value.
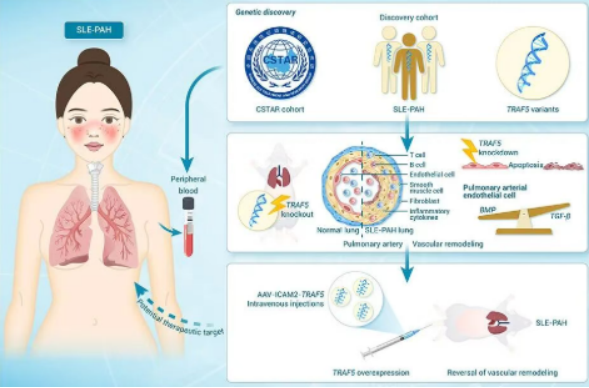
▲ Based on the CSTAR cohort, the research team discovered that TRAF5 variants aggravated SLE-PAH by mediating pulmonary vascular endothelial dysfunction.
This study is the first to establish rare TRAF5 variants as significant genetic risk factors for SLE-PAH. Through a multi-level systematic investigation - utilizing animal models, single-cell sequencing, and cellular experiments - the study elucidates the mechanism by which these variants mediate endothelial dysfunction via the BMP/TGF-β signaling pathway. The findings provide a novel biomarker for the early warning of SLE-PAH and lay a theoretical foundation for the development of targeted therapeutic strategies.
This study was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the National High Level Hospital Clinical Research Funding. Zhao Jiuliang, Li Mengtao, and Zeng Xiaofeng are co-corresponding authors. Deng Xiaoyue, Qian Junyan, and Wang Qian are co-first authors.
Written by and pictures courtesy of the Department of Rheumatology
Edited by Fu Tanping and Chen Xiao
Chief editor Duan Wenli
Supervised by Wu Peixin
