The team led by Dr. Han Bing, chief physician at PUMCH, has extensive experience in clinical and basic research on bone marrow failure diseases such as aplastic anemia (AA) and paroxysmal nocturnal hemoglobinuria (PNH) for years. Recently, the team has published a series of research findings in prestigious international journals including Blood Cancer Journal, Annals of Medicine, and British Journal of Hematology. These research findings can substantially enhance the clinical management of AA and PNH in China.
1. Blood Cancer Journal (Q1, IF=11.6)
(First author: Wang Leyu, direct-entry PhD student in Clinical Medicine, Class of 2023; corresponding author: Han Bing)

For patients with severe aplastic anemia (SAA) ineligible for hematopoietic stem cell transplantation, intensive immunosuppressive therapy (IST) with antithymocyte globulin (ATG) plus cyclosporine A (CsA) is the preferred treatment option. Thrombopoietin receptor agonists (TPO-RAs) such as avatrombopag (AVA) combined with ATG + CsA can enhance therapeutic efficacy. However, ATG is relatively expensive and associated with a relatively high incidence of adverse reactions, limiting its use in elderly patients.
The team conducted a multicenter prospective study in which 84 patients were randomized to receive either ATG + CsA + AVA or CsA + AVA. The objective response rates (ORRs) and complete response rates (CRRs) at 3, 6, and 12 months, as well as at the end of follow-up, showed no significant differences between the two groups (P > 0.05 for all, as shown in the figure below).
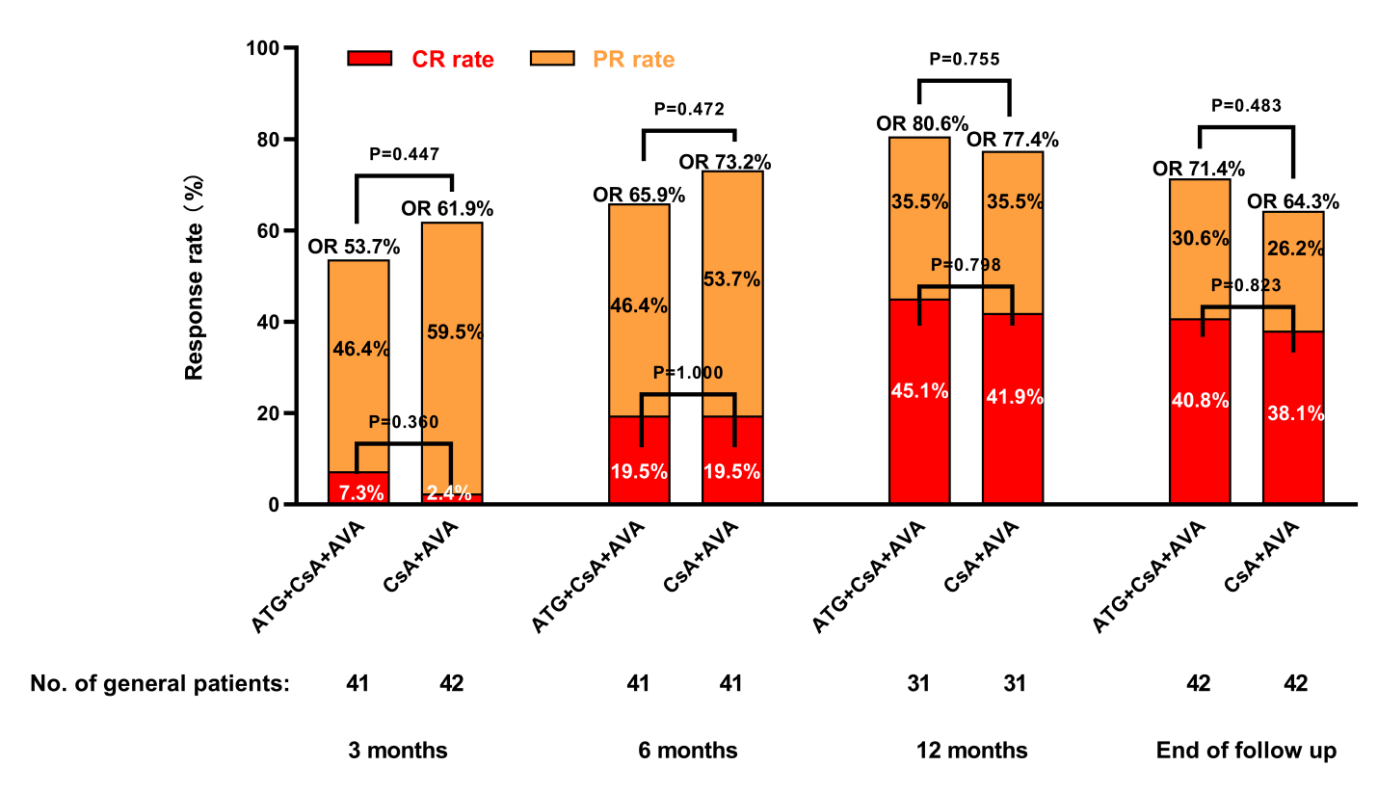
▲Efficacy comparison between ATG + CsA + AVA and CsA + AVA groups at different time points
Regarding safety, the incidence of adverse events in the ATG + CsA + AVA group was 64.3%, significantly higher than the 35.7% observed in the CsA + AVA group (P = 0.009). The two groups showed no statistical differences in long-term prognostic indicators including the rates of relapse (P = 0.667), death (P = 1.000), and clonal evolution (P = 1.000).
The team concluded that for older patients newly diagnosed with SAA, the CsA + AVA regimen not only shows comparable efficacy to the ATG + CsA + AVA regimen but also demonstrates a superior safety profile.
2. Annals of Medicine (Q1, IF=4.3)
(First author: Lin Xijuan, 8-year PhD student in Clinical Medicine, Class of 2020; corresponding author: Han Bing)

The treatment of refractory aplastic anemia (AA) patients who fail to respond to thrombopoietin receptor agonists (TPO-RAs) is a formidable challenge.
The team conducted a single-center retrospective study and selected 11 refractory patients diagnosed with AA between April 2023 and October 2023 who experienced treatment failure with immunosuppressive therapy (IST) and at least two other types of oral TPO-RAs. The researchers treated patients with high-dose romiplostim (ROM) (20 μg/kg/week), achieving an overall response rate of 72.7% with a median time to response of 1 month. At a median follow-up period of 8 months, the treatment demonstrated a good safety profile.
The study results indicate that in highly selected patients with severe AA who experienced treatment failure with TPO-RAs, the high-dose ROM has potential therapeutic value.
3. British Journal of Haematology (Q1, IF=3.8)
(First author: Zhang Li, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences; co-first author: Liu Ziwei, attending physician; corresponding authors: Zhang Fengkui, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences, and Han Bing)

Paroxysmal nocturnal haemoglobinuria (PNH) is a rare haematological disorder characterized by chronic intravascular haemolysis, anaemia, and thrombotic risk. The team conducted an open-label, randomized phase II proof-of-concept trial to evaluate the efficacy and safety of HRS-5965, a novel oral complement factor B inhibitor, in complement inhibitor-naïve PNH patients.
Twenty-six adult patients were randomized 1:1 to receive HRS-5965 50 mg orally twice daily (BID) (with potential up-titration to 100 mg BID depending on efficacy results) or 75 mg BID for 12 weeks.
In this study, both dosage groups of HRS-5965 demonstrated substantial therapeutic improvements. At week 12, patients receiving 50 mg BID and those receiving 75 mg BID experienced a mean haemoglobin (Hb) increase of 37.6 g/L and 37.7 g/L from baseline respectively; lactate dehydrogenase levels declined by 87% and 85% from baseline respectively. Transfusion independence was achieved in 100% of patients (12/12) in the 50 mg BID group and 83.3% of patients (10/12) in the 75 mg BID group. PNH red-cell clone size increased significantly, haptoglobin levels rose concurrently, and bilirubin and reticulocyte counts decreased. The drug demonstrated a favorable safety profile.
Both groups achieved >80% transfusion independence, confirming the potent efficacy of HRS-5965. HRS-5965 effectively controls the haemolytic process in PNH patients and shows promise as an important therapeutic option for future use.
Written by Wang Leyu
Pictures courtesy of Wang Leyu
Reviewed by Li Jian and Han Bing
Edited by Dong Jingge
Chief editor Duan Wenli
Supervised by Wu Peixin
