Recently, a collaborative team from PUMCH's Department of Hematology, led by Chief Physician Dr. Zhang Wei, and the Department of Dermatology, led by Chief Physician Dr. Liu Jie, published their latest research findings in the internationally prestigious journal JAMA Dermatology. This study focuses on cutaneous T-cell lymphoma, a rare and refractory skin malignancy, exploring a novel all-oral combination therapy that provides new insights for clinical treatment. This research was supported by the National Natural Science Foundation of China, the National High Level Hospital Clinical Research Funding, and the Beijing Natural Science Foundation, among others.
Cutaneous T-cell lymphoma (CTCL) is a malignant tumor that starts in T lymphocytes and primarily affects the skin. Common manifestations include skin rashes, plaques, swelling or scaling, which can easily be mistaken for chronic skin conditions like eczema or psoriasis in the early stages. Once it progresses to advanced stages, it may involve the blood, lymph nodes, and internal organs, severely affecting patients' quality of life and survival prognosis. Currently, treatment options for patients with advanced or repeatedly relapsed CTCL are limited, with short-lived efficacy and significant side effects. More effective and safer treatments are urgently needed.
This published study is the world's first prospective clinical trial that combines linperlisib, a PI3Kδ inhibitor, with chidamide, a histone deacetylase (HDAC) inhibitor for CTCL treatment. Both drugs are oral medications independently developed and marketed in China. This research validates their combined potential for treating CTCL, providing new therapeutic insights for clinical practice.
This was a Phase I single-arm clinical trial conducted at PUMCH, enrolling 22 patients with advanced CTCL who had repeatedly relapsed. Patients received oral combination therapy with linperlisib once daily and chidamide twice weekly, continuing until disease progression or intolerable toxic effects.
Regarding some primary and secondary outcomes, the objective response rate (ORR) was 59.1%: 13 patients showed significant tumor shrinkage, including 2 achieving complete response. The disease control rate (DCR) reached 86.4%: most patients experienced stable or improved condition. Adverse events were manageable, with the most common being nausea, pruritus, and skin rash, and no severe irreversible toxicity was observed.
The study also found that this regimen showed more significant efficacy in patients with earlier staging or treated with HDAC inhibitors for the first time, pointing direction for subsequent individualized treatment approaches.
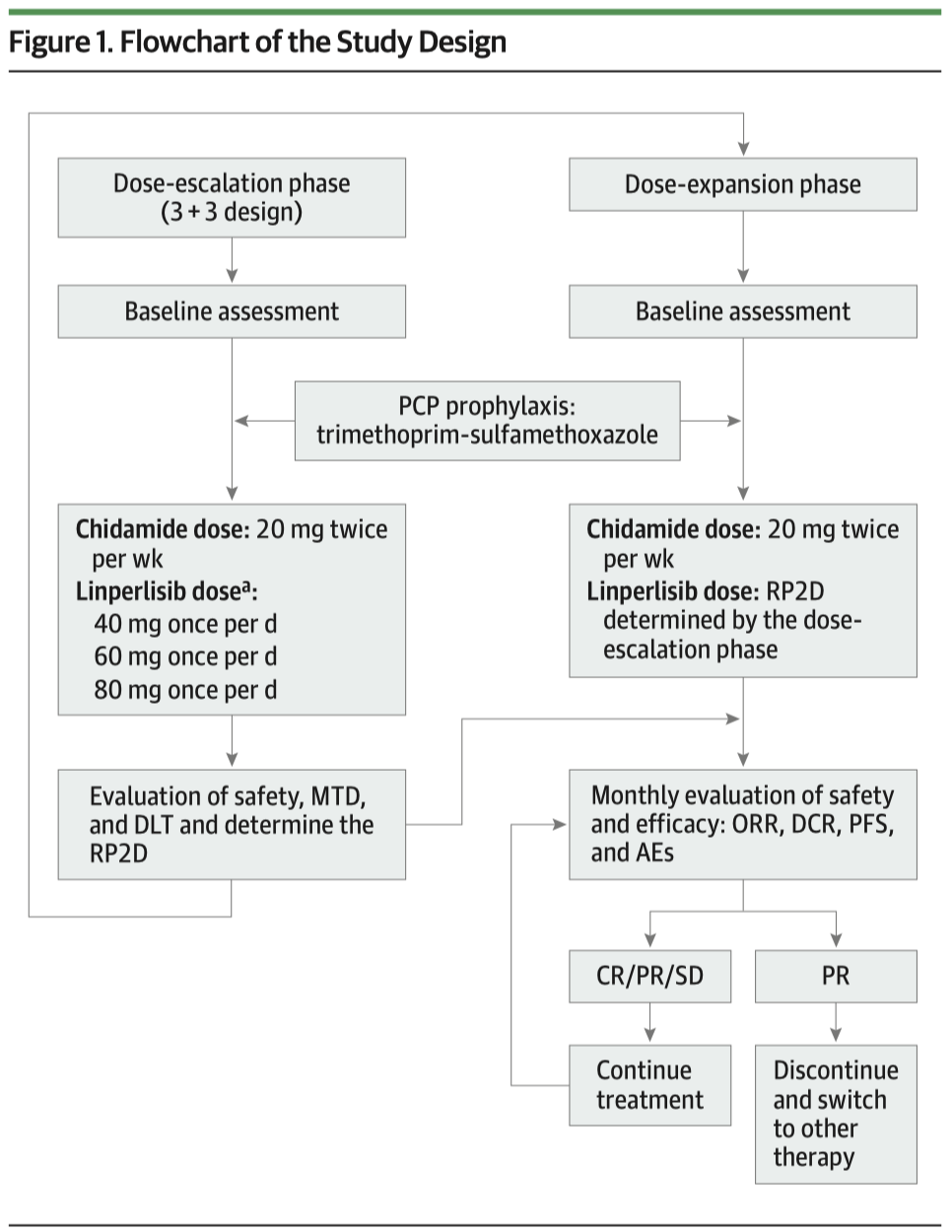
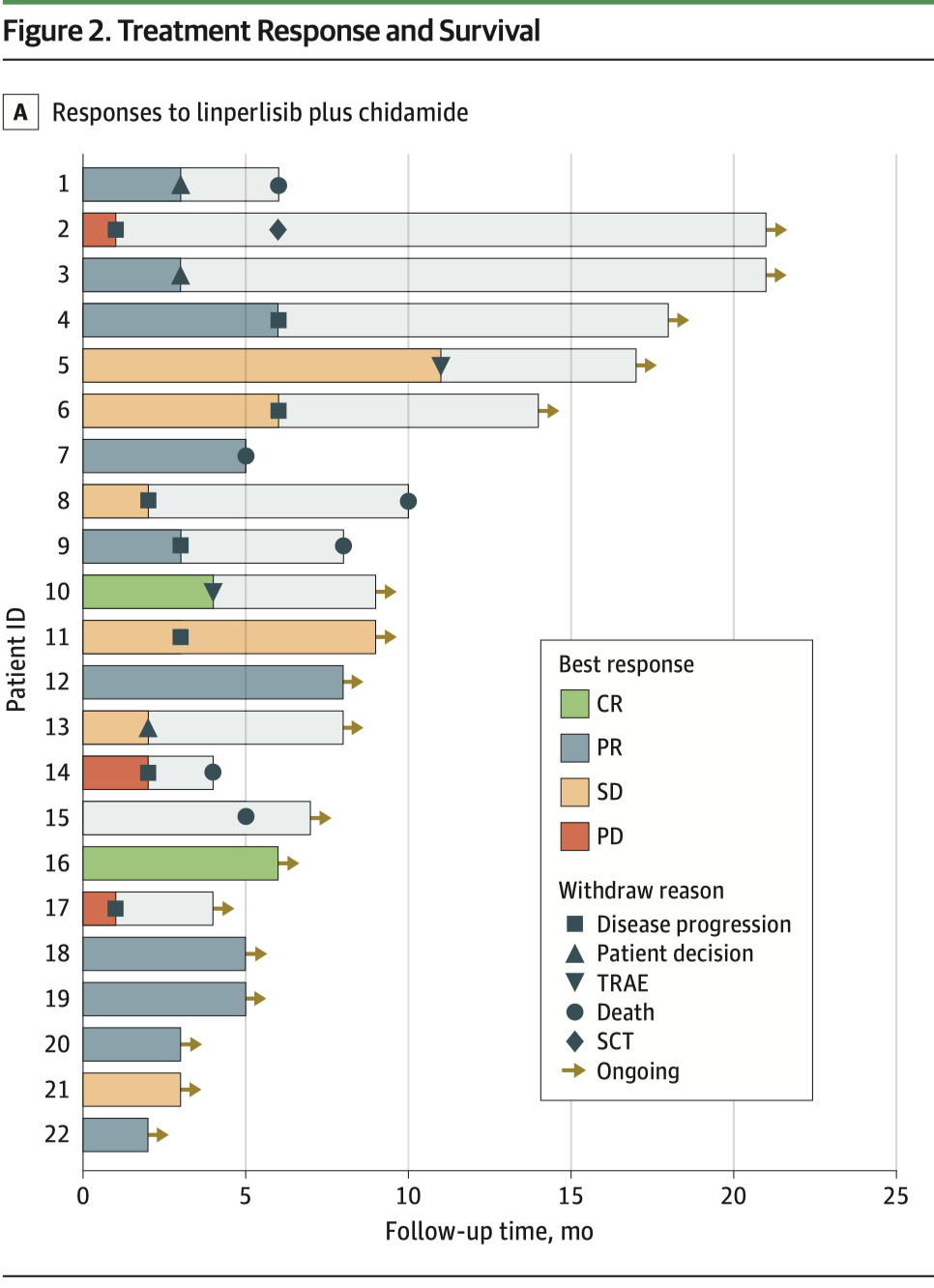
▲Left: Flowchart of the study design; Right: patient efficacy outcomes
This study provides a new treatment option for patients with relapsed or refractory CTCL—an all-oral regimen with confirmed efficacy and excellent safety profile. This regimen offers new hope particularly for patients who cannot tolerate chemotherapy or have had repeatedly relapsed.
Dr. Zhang Wei from the Department of Hematology, PUMCH and Dr. Liu Jie from the Department of Dermatology, PUMCH are co-corresponding authors for this paper. Dr. Pang Zhiyu from the Department of Dermatology, PUMCH is the first author.
The published article: Pang Z, Wang Y, Liu Z, et al. Linperlisib Plus Chidamide in Relapsed or Refractory Cutaneous T-Cell Lymphoma: A Nonrandomized Clinical Trial. JAMA Dermatol. Published online July 02, 2025. doi:10.1001/jamadermatol.2025.1926
Pictures courtesy of the Department of Dermatology
Edited by Chen Xiao and Wang Jingxia
