Recently, a collaborative study on the excretion pharmacokinetics of local anesthetics in breast milk among breastfeeding women, conducted by Associate Chief Physician Jing Quan from the Department of Dentistry and Researcher Wang Hongyun from the Clinical Pharmacology Center at PUMCH, was published in The Journal of the American Dental Association. The study found that after injection of 68 mg of articaine via supraperiosteal infiltration in breastfeeding women, the articaine concentration in breast milk peaked 15 minutes post-injection, but remained far below potentially toxic levels for infants, with extremely low safety risks for babies. For the vast majority of breastfeeding women, after receiving oral local anesthetic injection and completing routine dental procedures (typically lasting 15 minutes or longer), there is thus no need to discard their breast milk, and breastfeeding can be resumed immediately without risks.

Breastfeeding mothers often face a dilemma when receiving dental care: they fear that local anesthesia may "poison" their babies through breast milk, yet without anesthesia, they can hardly endure the pain of dental procedures. Many mothers simply choose to suffer through the dental pain without treatment. Articaine is currently the most commonly used local anesthetic in dentistry and has demonstrated good safety in adults, but its movement into breast milk and potential safety implications for infants remain unclear. Can mothers breastfeed immediately after local anesthetic injection? Should breast milk be expressed and discarded? Is a temporary cessation of breastfeeding necessary? Due to the lack of clinical research evidence, international guidelines and consensus statements have provided only "vague" recommendations, leaving dental practitioners without authoritative answers, with some dentists reluctantly declining to treat breastfeeding mothers.
To address this clinical need, the Department of Dentistry and the Clinical Pharmacology Center at PUMCH initiated this single-center, randomized, open-label clinical study. The research recruited 12 breastfeeding mother volunteers. Combining clinical pharmacology techniques and methods with clinical practices, the research team used liquid chromatography-tandem mass spectrometry to measure articaine concentrations in breast milk samples at different time points following supraperiosteal infiltration injection of 68mg articaine, exploring the excretion pharmacokinetic properties of this local anesthetic. In addition, the study employed physiologically based pharmacokinetic (PBPK) modeling to simulate drug exposure in breastfed babies and assess the safety risks of breastfeeding after injection.
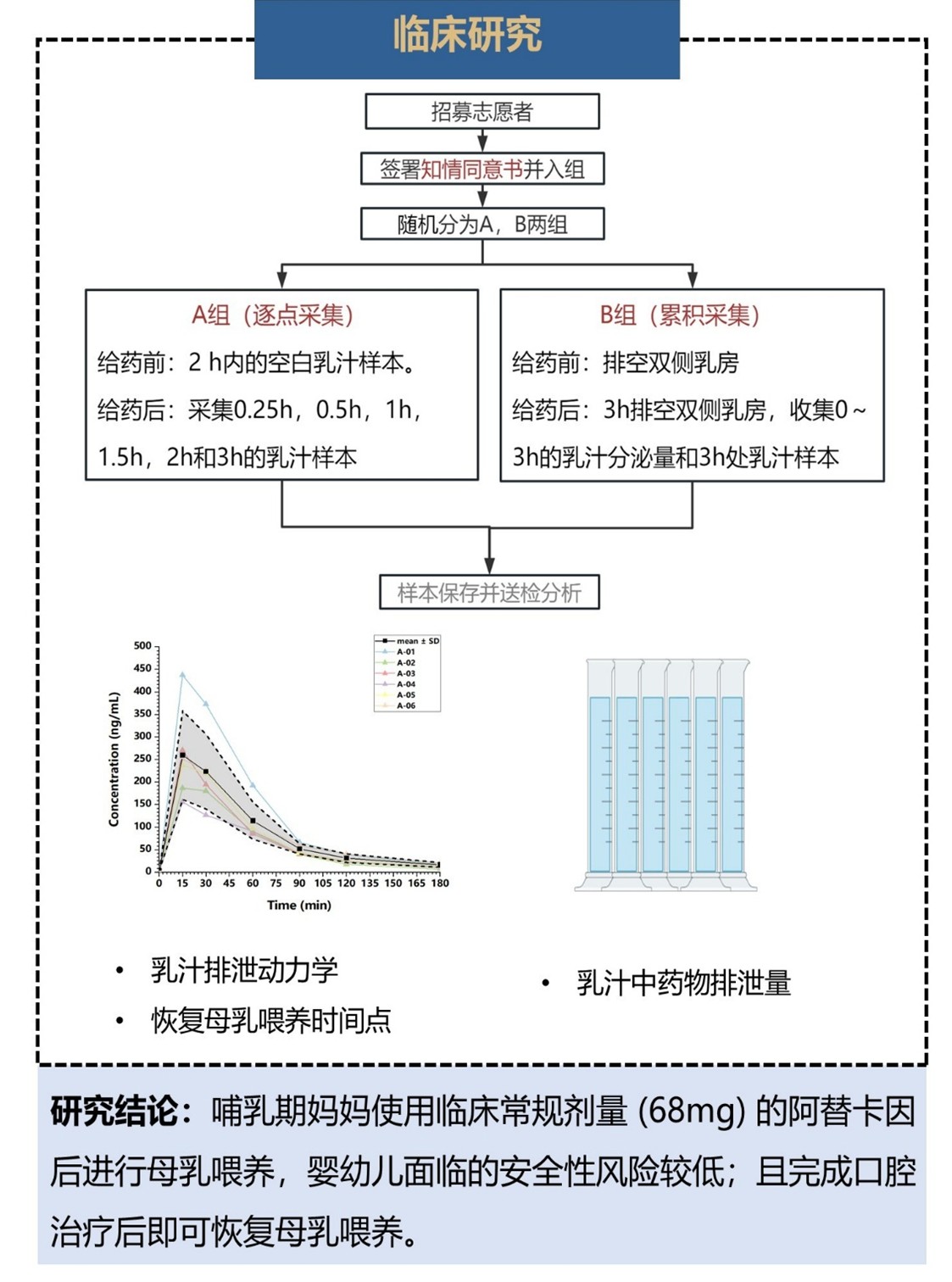
The study results showed that breastfeeding mothers using clinical standard dosage (68mg) of articaine pose low safety risks to infants through breastfeeding, and breastfeeding can be resumed immediately after completing dental treatment. This research not only resolves the dilemma for breastfeeding mothers but also provides scientific evidence for dental healthcare providers to offer comfortable, pain-free treatment to them. These findings have been cited by LactMed, the internationally authoritative database for medication safety during lactation.
Link to the published article
https://jada.ada.org/article/S0002-8177(25)00217-X/fulltext
