Recently, the team led by Director Li Mengtao from the Department of Rheumatology at PUMCH, in collaboration with Researcher Yang Jun's team from Zhejiang University, revealed the dual mechanisms of the Activin A/ALK4 signaling pathway in Th17 cells and pulmonary vascular endothelial cells, providing a new therapeutic target for systemic lupus erythematosus-associated pulmonary arterial hypertension. This research was published in the prestigious international journal Arthritis & Rheumatology.
Pulmonary arterial hypertension (PAH) is a severe complication and one of the major causes of death in systemic lupus erythematosus (SLE). Current treatment approaches are mainly pulmonary vasodilators, but they can fail to fundamentally block or even reverse the core disease process. Elucidation of the pathogenic mechanisms of SLE-associated PAH (SLE-PAH) and precise identification of intervention targets are crucial levers to pull for improving patient prognosis.
To address this urgent clinical need, the research team first performed mass cytometry to identify the major affected immune cell population after the treatment in SLE-PAH patients. The researchers found that CD4+ T cells in peripheral blood were significantly reduced after treatment. Further flow cytometric analysis revealed that the proportion of pro-inflammatory Th17 cells in patients' peripheral blood was abnormally elevated. Serological testing showed that IL-17 and Activin A levels in SLE-PAH patients were significantly higher than those in patients with SLE alone and healthy individuals, and their elevation correlated strongly with core clinical indicators of PAH. These findings provide important clues for the pathogenic hypothesis.
The research team constructed a hypoxic mouse model with CD4+ T cell depletion. Results confirmed that depleting CD4+ T cells could effectively suppress pulmonary arterial pressure elevation but could not reverse established pulmonary vascular structural remodeling, clarifying the causative role of CD4+ T cells in pulmonary arterial hypertension development and their therapeutic limitations.
The researchers further conducted Th17 cells coculturing with pulmonary vascular endothelial cells. The study found that when Th17 cells overexpressed ALK4, the receptor for Activin A, they could potently induce endothelial-to-mesenchymal transition (EndoMT) in endothelial cells, which is the core pathological process of pulmonary vascular remodeling.
By constructing an ALK4-overexpressing SLE-PAH mouse model, the research team discovered that Activin A exerts “pathogenic effects” through its receptor ALK4 in both Th17 cells and pulmonary vascular endothelial cells. The dual mechanisms simultaneously exacerbate inflammatory responses and directly drive vascular structural destruction, thereby promoting the onset and progression of SLE-PAH. This finding provides an important theoretical foundation for subsequent research and clinical intervention.
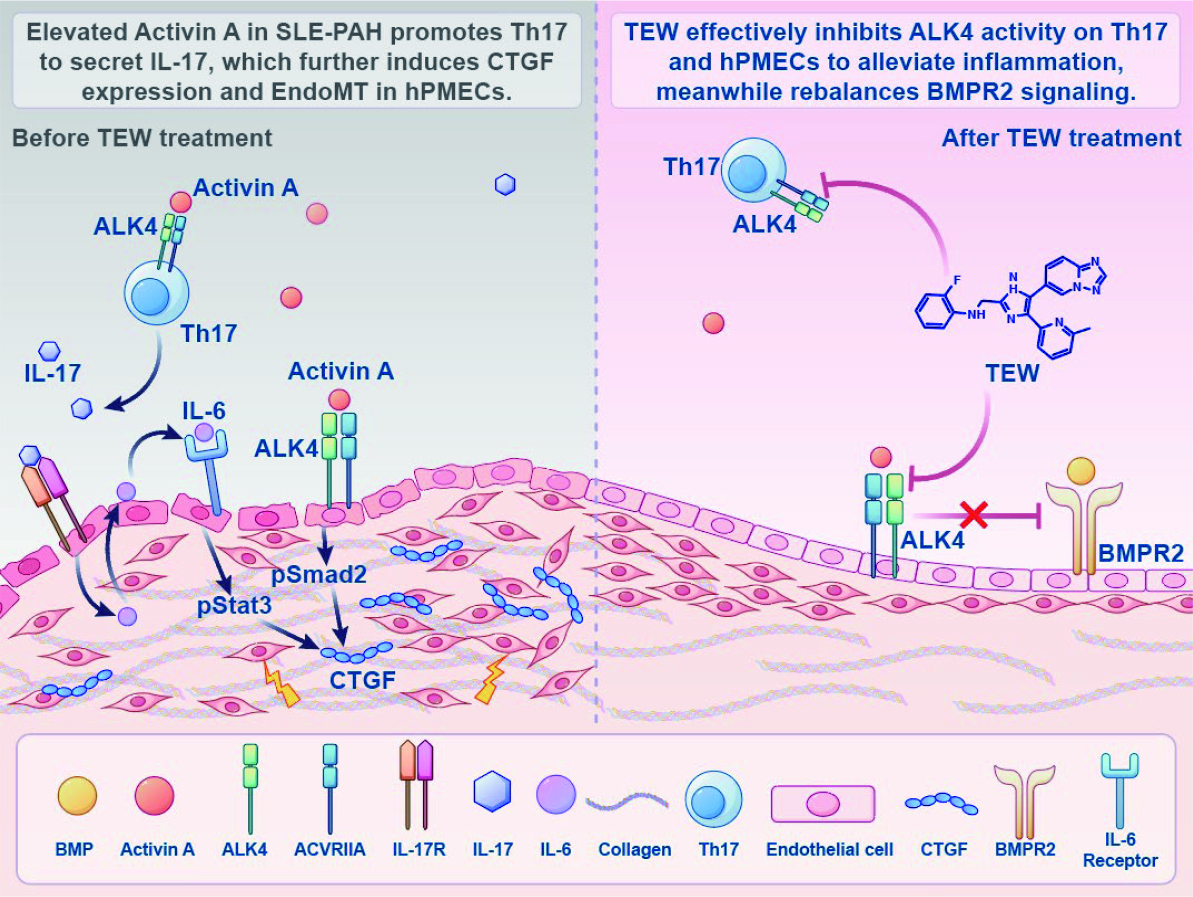
This study explored another novel pathogenic mechanism of SLE-PAH, revealing the central role of the Activin A/ALK4 signaling pathway in SLE-PAH and its unique dual-target mechanism, providing new perspectives for understanding the pathogenesis of connective tissue disease-associated PAH. Intervention strategies targeting this pathway are expected to simultaneously suppress excessive immune responses and block vascular structural remodeling, thereby "killing two birds with one stone". Such strategies hold promise for breaking through current therapeutic bottlenecks and significantly improving patient prognosis.
Co-corresponding authors of this paper are Researcher Yang Jun from the Department of Physiology, School of Basic Medical Sciences, Zhejiang University, and the State Key Laboratory of Transvascular Implants, the Second Affiliated Hospital, Zhejiang University School of Medicine; and Director Li Mengtao from the Department of Rheumatology, PUMCH. The co-first authors are Jing Shuliang from the Department of Physiology, School of Basic Medical Sciences, Zhejiang University, and Attending Physician Qian Junyan from the Department of Rheumatology, PUMCH.
Written by and pictures courtesy of the Department of Rheumatology
Edited by Fu Tanping and Chen Xiao
Link to the original article
https://www.medrxiv.org/content/10.1101/2025.01.23.25321014v1.article-metrics
