Recently, the liver surgery team at PUMCH, in collaboration with several research institutes, made two major breakthroughs on artificial livers. The teams used embedded bioprinting to construct artificial liver with hepatic vein structures, and acoustical holographic lattice-based acoustic tweezers to pattern hepatocytes with superior viability and functionality. These discoveries create opportunities for alternative liver transplant donor options and enhance the speed and effectiveness of tissue engineering. Both of these research breakthroughs were published as original articles in the renowned journal “Biomaterials”.
In recent years, in vitro tissue engineering has emerged as a crucial research direction in regenerative medicine. While it has made significant progress in creating planar and tubular tissues, there are still certain limitations. Organoids and microfluidic chips demonstrate efficacy in fabricating micro-scale tissues, yet encounter challenges in simulating complex organ functions. Conversely, while 3D bioprinting facilitates the production of expansive biomimetic tissue frameworks, its extrusion-based printing method is prone to tissue damage, and its printing velocity
Human organs possess intricate functional and structural complexities. For instance, the adult liver, the largest organ in the human body, comprises approximately 500,000 to 1 million hexagonal liver lobules housing around one trillion cells, which are arranged in a dense, interconnected system of blood vessels and bile ducts, enabling the execution of over 500 vital physiological functions encompassing metabolism, synthesis, and detoxification. Given such complexity, several challenges arise in the development of artificial livers: How to construct a vascular network with active tissue function, how to make artificial livers functional, and how to ensure their right size.

The liver surgery team of Mao Yilei and Yang Huayu from PUMCH, in collaboration with Ye Hua’s team from the Institute of Biomedical Engineering, University of Oxford/Oxford Suzhou Centre for Advanced Research, and Huang Pengyu’s team from the Institute of Biomedical Engineering, Chinese Academy of Medical Sciences/Tianjin Institute of Medical and Health Sciences, printed hepatospheroid-encapsulated-artificial livers (HEALs) using an “omnidirectional printing embedded network (OPEN)” as a novel support medium. Researchers transplanted HEALs into mice, and substantial angiogenesis of the transplant was observed after two weeks.
Compared with traditional 3D-printed livers, HEALs offer significant improvements, enabling centimeter-scale tissue construction and demonstrating significant advantages in liver function and post-transplantation angiogenesis. This technique opens up potential pathways for drug screening, mechanism researchand liver transplant alternatives.
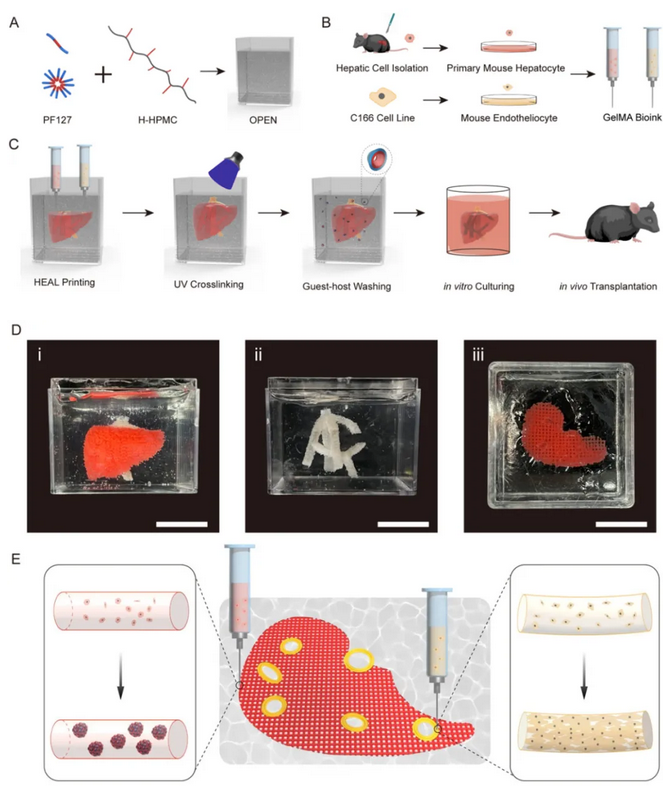
▲Embedded bioprinted liver in OPEN with predesigned hepatic vein structures based on primary mouse hepatocytes (PMHs) and mouse endothelial cells C166
C
Concurrently, Mao Yilei and Yang Huayu’s team collaborated with Li Fei and Zheng Hairong’s team from the Institute of Biomedical and Health Engineering, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, to construct in vitro biomimetic 3D liver models using “acoustical holographic lattice-based acoustic tweezers”
Acoustic holographic tweezer is a technique that utilizes acoustic techniques for capturing, assembling, manipulating, and screening cells. The research team describes it as “manipulating objects from a distance”. However, this technique has two major limitations: first, it can only create linear-outline patterns, which limits the range of biological model structures it can make; second, the impact of this technique on cell viability and function is not yet clear.
In response to these challenges, the research team implemented targeted modifications to enhance the intelligence of acoustic holographic tweezers. The team found that compared to traditional 3D culture models, acoustic holography-patterned primary hepatocytes exhibited a large number of self-assembled spheroids within 1 minute. This process is not only rapid, but also highly effective. Following in vitro culture, the spheroids demonstrated significant increases in diameter and notably enhanced core functions such as protein synthesis, glucose metabolism, and detoxification. The team stated that the “acoustical holographic lattice-based acoustic tweezer” is a non-contact and non-invasive technique capable of precise and flexible manipulation and rapid patterning. These unique strengths underscore its great potential in constructing in vitro 3D models.

▲Viability and function of primary mouse liver tissue constructed using acoustic holographic lattice-based acoustic tweezers
Written by Yang Huayu and Gan Dingzhu
Edited by Gan Dingzhu and Chen Xiao
Translated by Liu Haiyan
Reviewed by Xu Haifeng and Wang Yao
