Recently, Professor Zeng Xiaofeng and Professor Leng Xiaomei’s team from the Department of Rheumatology at PUMCH had their research published in the top-tier journal “THE LANCET Rheumatology” (IF: 25.4), which is “comparing tocilizumab biosimilar BAT1806/BIIB800 with reference tocilizumab in patients with moderate-to-severe rheumatoid arthritis with an inadequate response to methotrexate: a phase 3, randomized, multicenter, double-blind, active-controlled clinical trial”. BAT1806/BIIB800 showed equivalent efficacy, safety and immunogenicity as reference tocilizumab. The research contributed to the approval of BAT1806/BIIB800 in China and the US, providing a new treatment option for patients with rheumatoid arthritis.

Rheumatoid arthritis (RA) is a disabling, incurable autoimmune disease, and its treatment requires lifelong management. For RA patients who do not respond well to conventional disease modifying anti-rheumatic drugs (DMARDs), long-term use of biologics is often necessary. Tocilizumab is an immunoglobulin G1 monoclonal antibody that targets the receptor for interleukin-6 (IL-6), and its monotherapy has shown superior efficacy over tumor necrosis factor inhibitors (TNFi). With greater clinical therapeutic benefits, tocilizumab is currently the preferred biologic agent for patients with TNFi-refractory RA. However, the high cost of imported tocilizumab limits its widespread use.
Led by Prof. Zeng Xiaofeng, this international multicenter clinical study enrolled a total of 621 patients with moderate-to-severe active RA who had an inadequate response to methotrexate. The study was conducted at 54 research centers across five countries (China, Ukraine, Poland, Georgia and Bulgaria), with a total treatment duration of 48 weeks.
The study consisted of two treatment periods: TP1 (Weeks 0 to 24) and TP2 (Weeks 24 to 48). During the TP2 phase, the research team innovatively designed a medication switch (50% of the control group switched to BAT1806/BIIB800) to further confirm the drug’s equivalence. In addition, when defining the equivalence margins, the study factored in the different requirements of regulatory authorities in Europe, the United States, and China.
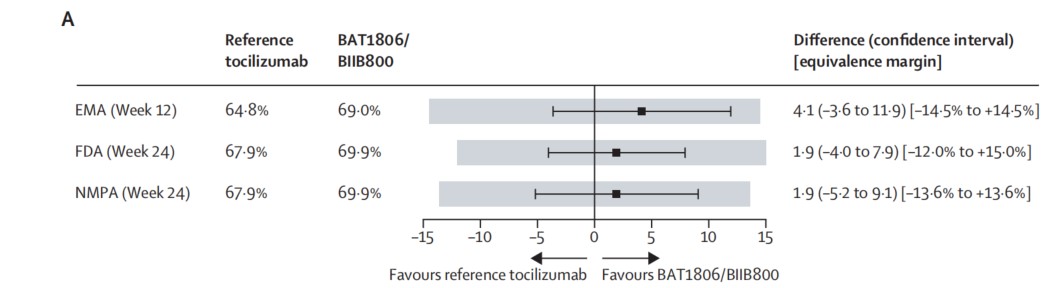
▲The ACR20 time points and equivalence margins allowed by regulators. The gray boxes represent the equivalence margin allowed by the corresponding regulatory agency.
Note: ACR20 refers to an improvement of more than 20% in disease activity based on the American College of Rheumatology criteria. EMA: the European Medicines Agency; FDA: the U.S. Food and Drug Administration; NMPA: the National Medical Products Administration of China
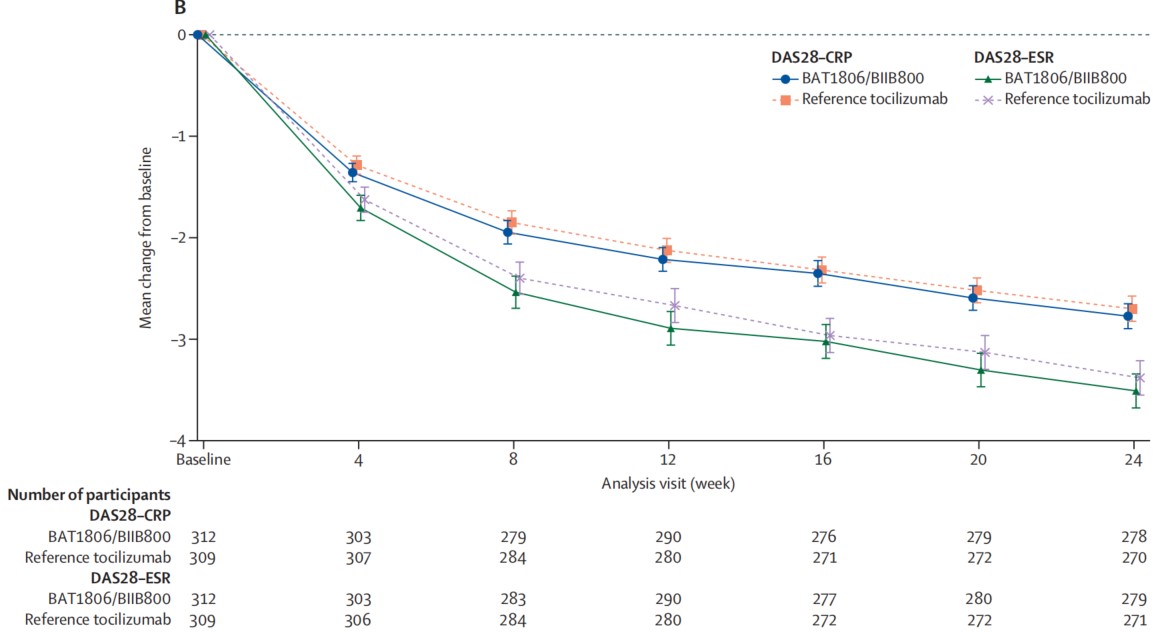
▲The average change over time (95% CI) of DAS28-ESR and DAS28-CRP relative to baseline in TP1 (Treatment Period 1) in the Full Analysis Set
Note: DAS28-ESR: the 28-Joint Disease Activity Score using erythrocyte sedimentation rate; DAS28-CRP: the 28-joint Disease Activity Score using C-reactive protein
The study results showed that BAT1806/BIIB800 demonstrated similar ACR20 response rates to reference tocilizumab at Week 12 and Week 24 within the predefined equivalence margins of regulatory agencies in Europe, the United States, and China. The average change over time (95% CI) of DAS28-ESR and DAS28-CRP relative to baseline indicated that BAT1806/BIIB800 was highly similar to reference tocilizumab in alleviating disease activity.
This study demonstrated the equivalence of BAT1806/BIIB800 with reference tocilizumab in terms of clinical efficacy, safety, and immunogenicity. Thanks to the research, BAT1806/BIIB800 became the first biosimilar of its kind that was approved by the NMPA in China (January 2023), as well as the first monoclonal antibody drug approved by the U.S. FDA and granted market authorization in the United States (September 2023).
“The LANCET Rheumatology” also published in the same issue a comment “Biosimilar tocilizumab—better access and lower cost?”, which spoke highly of the study’s design and findings.
Written by and pictures courtesy of the Department of Rheumatology
Edited by Yan Xiaobo and Chen Xiao
Translated by Liu Haiyan
Reviewed by Jiang Nan and Wang Yao
