On November 6, 2023, the research paper on the global and Chinese trends in the development of drugs for rare diseases, a collaboration between Professor Zhang Shuyang, the President of PUMCH, Associate Research Fellow Chen Rui, and Professor Chen Xiaoyuan from Tsinghua University, was published in the internationally prestigious Nature Reviews Drug Discovery (a Tier 1 journal, or among the top 5%, as ranked by the Chinese Academy of Sciences, IF: 120.1). The research conducted a breakdown analysis of rare disease drugs in development by stages and therapeutic areas, calculated the proportion of different types of drugs, and the drivers behind industry-academia-research collaborations. From those perspectives, the research compared the global and Chinese rare disease drug development to shed light on the similarities and differences and identify the weaknesses, growth points, and driving forces in the Chinese endeavors. This study provided, for the first time, a macro-level comparison of rare disease drug pipelines between China and overseas. It aims to identify unmet clinical needs in China, explore advantageous areas for growth, and provide data support for target discovery, policy-making, and investment planning for rare disease drug development. In a nutshell, the purpose is to provide a scientific basis for advancing rare disease diagnosis, treatment, and research in China.

The team conducted extensive data collection, screening, and analysis. To address discrepancies in inclusion criteria for rare diseases between China and foreign countries, the team adopted the rare disease definitions of the United States or the European Union and incorporated them into the global database; the Chinese database included not only the first and second batches of rare disease catalogs but also indications that are currently searchable and meet regional standards for incidence or prevalence. This way, comparable domestic and global database were established.
The study showed that over the past five years, the number of rare disease drugs under development in China has significantly increased, with an average annual growth rate of 34%, which surpasses the global growth rate by nearly 42 percentage points. The growth period coincides with the implementation of favorable policies for pharmaceutical research and development since 2017. As of the end of 2022, there were a total of 840 rare disease drugs under development in China, with the proportion in the preclinical, Phase I, Phase II, Phase III, and pre-registration stage being 49%, 25%, 18%, 7%, and 2% respectively. In comparison, as of the end of 2022, the global total was 5,215, distributed across different stages of development in similar proportions to China.
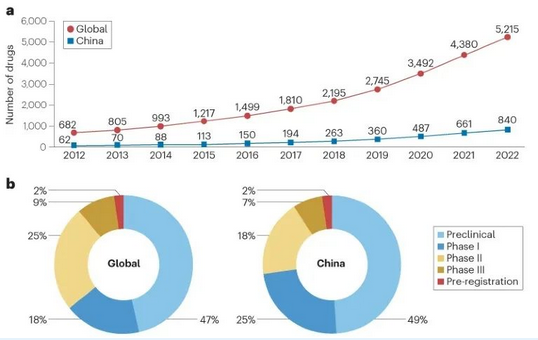
▲a. The number of rare disease drugs in preclinical and clinical pipelines globally and in China from 2012 to 2022. b. The breakdown of rare disease drugs in the pipeline by development stage globally and in China, as of December 31, 2022
A breakdown by therapeutic area shows that oncology drug development efforts are the most active both globally and in China, accounting for 43% of the pipeline globally and 71% in China. Following oncology, other therapeutic areas ranked by their proportion of drugs in the pipeline globally are neurological diseases (12%), respiratory diseases (8%), digestive/metabolic diseases (7%), and immune system diseases (6%). The distribution in China differs somewhat, with only 4% of all drugs under development targeting neurological diseases and 2% targeting digestive/metabolic diseases. This suggests broad unmet clinical needs in these therapeutic areas. In recent years, with the support of the national rare disease registry system, clinical cohorts for rare neurological and metabolic diseases have been established successively, continuously collecting information to drive drug development in relevant fields.
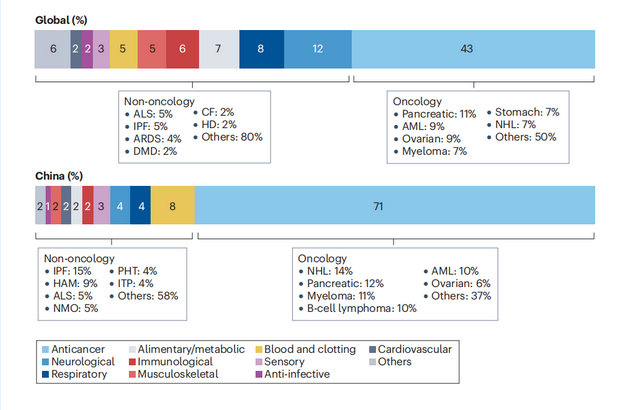
▲The distribution of rare disease drugs in various therapeutic areas globally and in China
Globally, biologics account for nearly half (49%) of the rare disease drug development pipeline, with the top three categories being protein-based drugs (20%), cell-based drugs (16%), and nucleic acid-based drugs (9%). In China, nucleic acid-based drugs only account for 3%. Considering the rapid development of nucleic acid-based drugs and their advantages in rare diseases, they may represent a growth area for rare disease drug development in China.
The study also compared the industry-academia-research collaboration in rare disease drug development globally and in China. In the global context, academic institutions are most engaged in the R&D of chemical drugs, while in China, it is cell-based drugs. This reflects China’s driving force advantage in cellular and gene therapy and that early-stage industry-academia-research collaboration holds the key to early translation.
The low investment return of rare disease drug development makes it crucial to accurately identify needs and plan scientifically to avoid homogenous competition. In the past decade, China has introduced multiple policies on rare disease drug development, providing solid policy support for promoting the high-quality development of rare disease-related initiatives. This study conducts precision analysis of drug development targets, dissects China’s strengths and weaknesses in drug development and thoroughly examines R&D drivers that China can draw upon. This provides a necessary scientific basis for mapping out R&D priorities as well as coordinating the allocation of research and development resources. According to Professor Zhang Shuyang, driven by sustained policy incentives and regulatory reforms, the future of rare disease drug development in China will be promising.
Written by Chen Rui and Chen Xiao
Translated by Liu Haiyan
Reviewed by Chen Rui and Wang Yao
