Recently, a collaborative team led by Li Yongzhe, Researcher in the Department of Clinical Laboratory, PUMCH; Zheng Wenjie, Chief Physician in the Department of Rheumatology, PUMCH; Liu Yudong, associate researcher at the National Center for Clinical Laboratories; and partners at the National Center for Protein Sciences have successfully applied, for the first time globally, artificial intelligence-driven proteomics to construct a diagnosis and stratification model for Behçet's disease (BD). This breakthrough offers new strategies for early diagnosis and precision treatment of the condition. The findings were published in Advanced Science (IF=14.1), a leading international journal.

Behçet's disease (BD) is a chronic multisystem inflammatory disorder characterized by a heterogeneous spectrum of clinical manifestations. The absence of disease-specific blood biomarkers has long hindered clinical stratification and treatment efficacy assessment.
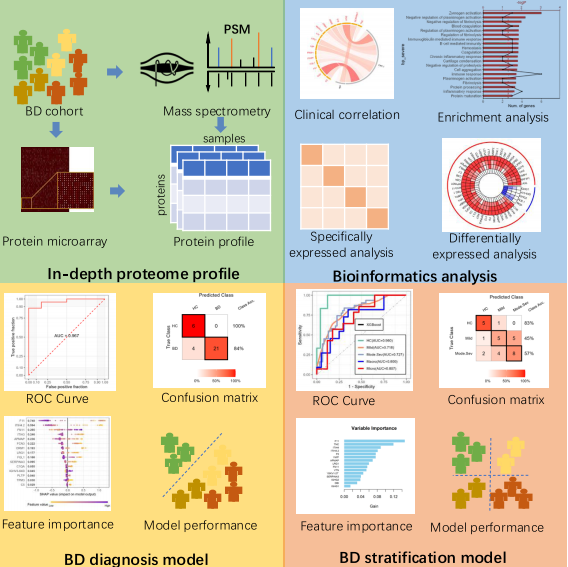
▲Figures: “AI+proteomics” approach for developing BD diagnosis and stratification models
The study identified dozens of proteins with critical roles in BD pathogenesis, including complement component C4B. Protein-protein interaction (PPI) network analysis identified C4B as the hub protein with the highest degree centrality, suggesting a potentially critical role in disease progression. Functional annotation indicated that severity-associated upregulated proteins were primarily involved in protein activation cascades, complement activation, and humoral immune responses. In the study, FXI (coagulation factor XI) emerged as the top-ranked feature in both diagnosis and stratification models, suggesting a potentially important role in the vasculitic mechanisms of BD.
This study demonstrates the power of "AI-driven proteomics" in constructing high-precision diagnosis and stratification models. The findings reveal complex interactive networks among infection, complement activation, coagulation dysfunction, and immune-inflammatory responses in BD pathogenesis—providing a crucial theoretical foundation for developing targeted therapies and personalized intervention strategies. With ongoing multicenter, prospective studies, this model has the potential to translate into a practical clinical tool, facilitating early diagnosis, timely treatment initiation, and comprehensive disease management of BD.
Co-first authors are Cheng Linlin, Resident Physician in the Department of Clinical Laboratory, PUMCH; Li Mansheng, Associate Researcher at the National Center for Protein Sciences. Corresponding authors are Researcher Li Yongzhe, Chief Physician Zheng Wenjie, and Associate Researcher Liu Yudong.
