Recently, the team led by Jin Hongzhong, Director of the Department of Dermatology at PUMCH, published their latest research in the internationally prestigious British Journal of Dermatology (IF=9.6). For the first time, the study systematically revealed the core driving role of the IL-36 signaling pathway in the pathogenesis of Sweet syndrome (SS) and its feasibility as a therapeutic target, offering innovative treatment approaches for refractory SS cases. This project was supported by the National High Level Hospital Clinical Research Funding.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, typically presents with severely painful edematous erythematous plaques, often accompanied by high fever, and may be associated with underlying conditions such as hematologic diseases and inflammatory bowel disease. It is commonly treated with high-dose systemic corticosteroids, but some patients are resistant to corticosteroids. To date, the pathogenesis and core inflammatory pathways of this disease remain unclear.
In the early phase, the research team successfully treated corticosteroid-resistant or contraindicated SS patients with an IL-36R inhibitor, achieving significant therapeutic effects. The team combined single-cell spatiotemporal transcriptomics sequencing with multiplex immunofluorescence to conduct longitudinal analysis of skin lesion tissues and peripheral blood samples from patients before and after the treatment. The results revealed a distinct neutrophil subpopulation within the skin lesions that releases neutrophil extracellular traps (NETs) and elastase, which cleaves and activates IL-36 precursor proteins, thereby forming an inflammatory signal amplification loop.
Spatial transcriptomics further revealed that these neutrophils form localized "inflammatory hotspots" with keratinocytes at the dermoepidermal junction, maintaining an abnormal skin state. Following treatment with the IL-36R inhibitor, this inflammatory network was significantly weakened, and both neutrophil activation and IL-36 signaling were rapidly reduced.
The study also found that CD8⁺ T cells, together with keratinocytes and neutrophils, form an immune-inflammatory chain. Based on these findings, the team proposed that keratinocytes, heterogeneous neutrophils, and CD8⁺ T cells constitute an inflammatory loop that drives the occurrence and progression of SS. Specifically, elastase released by neutrophils mediating IL-36 activation represents a pivotal triggering event.
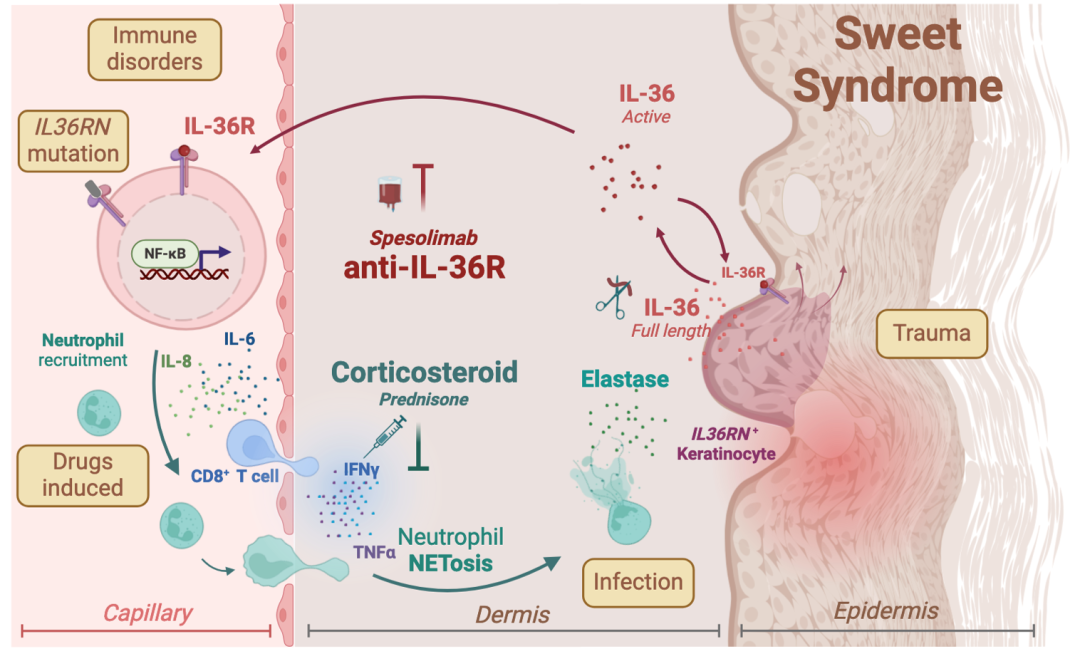
▲Inflammatory loop underlying the occurrence and progression of SS
This study systematically elucidated the immune-inflammatory mechanisms of SS at the molecular, cellular, and spatial levels, and validated the feasibility of the IL-36 signaling pathway as a therapeutic target. IL-36R inhibitors are expected to become a novel precision treatment option for patients resistant to or with contraindications to corticosteroids and provide important theoretical basis and experimental support for intervention strategies in other refractory neutrophilic dermatoses.
The corresponding authors of the article are Jin Hongzhong, Director of the Department of Dermatology at PUMCH, and Associate Professor Chen Wei from the School of Biological Science and Medical Engineering at Beihang University. The first authors are Wu Chao, attending physician, and Pang Zhiyu, resident physician, both from the Department of Dermatology at PUMCH.
Written by and pictures courtesy of the Department of Dermatology
Edited by Wang Jingxia
