Professor Chen Limeng’s team from the Department of Nephrology, PUMCH recently published an article in “Cell Death & Disease” (a Tier 1 journal, or among the top 5%, ranked by the Chinese Academy of Sciences), which demonstrated for the first time that acetaldehyde dehydrogenase 2 (ALDH2) protects renal tubular structure and function by stabilizing peroxisomal proliferator-γ coactivator-1α (PGC-1α), a key mitochondrial protein. This finding sheds new light on the clinical evaluation and prevention of acute kidney injury and transporter dysfunction. The study received funding support as a major project under the Science & Technology Innovation 2030 and the National High Level Hospital Clinical Research Funding.

Acute kidney injury (AKI) is a clinically common syndrome of sudden decline in renal function within a short space of time (hours to days), with various causes. AKI is an independent risk factor that prolongs patients’ hospital stay, increases costs and the odds of death, but effective measures for its early diagnosis and intervention are lacking. Proximal tubular injury and programmed death are the most common structural basis of AKI, and the tubular transporter dysfunction is also the etiology of several inherited and acquired rare transporter diseases (Fanconi syndrome).
The renal tubular epithelial cells are responsible for reabsorbing more than 90% of water, salts and small molecules in primary urine via an active transport process that requires a large amount of energy, highly dependent on mitochondrial energy supply. ALDH2 is a key enzyme that is involved in alcohol metabolism and mitochondrial oxidative ATP production. Its mutation is common among East Asians, constituting the genetic cause for “Asian flush” after alcohol consumption. ALDH2 is also an important factor that affects the efficacy of nitrates in treating coronary heart diseases, however its role in mitochondrial homeostasis in cases of AKI is unclear.
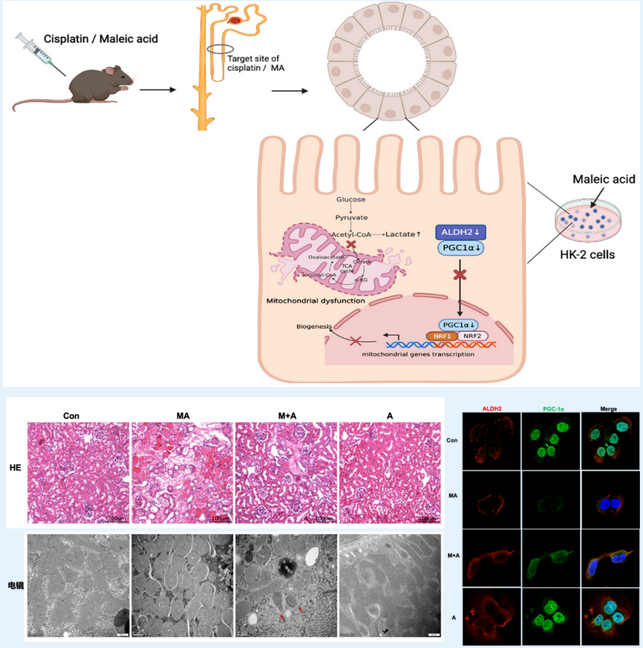
▲Overall schematic diagram of the study: activation of ALDH2 attenuates maleic acid-induced kidney injury and improves mitochondrial structure and function; ALDH2 modulated nuclear translocation of PGC-1α
The study found that ALDH2 expression was predominantly decreased in multiple models of AKI and Fanconi syndrome both in vivo and in vitro. ALDH2 knockout mice exhibited exacerbated kidney impairment and apoptosis of tubular epithelial cells. In contrast, ALDH2 activation alleviated AKI. This confirms that reduced expression of ALDH2 holds the key to the occurrence of AKI and Fanconi syndrome.
The research team further designed exploratory experiments of different dimensions and types, thereby ascertaining mitochondrial biogenesis as the key pathway for modulation by ALDH2.
Transcriptomics revealed that the oxidative phosphorylation pathway was positively enriched in the renal tissues after Alda-1 pre-treatment in MA-induced mice. In vitro cell experiments revealed that ALDH2 activation restored mitochondrial structure, mitochondrial membrane potential, and respiration rate, but downregulated glycolysis, thereby reducing cell damage. Co-immunoprecipitation assays revealed that ALDH2 interacted with PGC-1α, a master regulator of mitochondrial biogenesis, and advanced its nuclear translocation. Subsequently, in in-vitro cell experiments, PGC-1α knockdown almost abolished the improvement of ALDH2 activation on renal tubular epithelial cells. This confirms that PGC-1α is a key downstream molecule for the regulation of ALDH2.
Thus, the study confirmed for the first time that ALDH2 activation alleviated mitochondrial dysfunction in AKI by enhancing PGC-1α-mediated mitochondrial biogenesis. Hence, ALDH2 may act as a potential therapeutic target to prevent AKI progression.
On the basis of the above research, the team further carried out a series of work around ALDH2 and kidney: in collaboration with Prof. Xu Tengda from the Department of Health Management and Prof. Qiu Ling’s team of the Department of Clinical Laboratory, the research team made an initial observation of the relationship between common male mutations of ALDH2 and kidney function in 15,000 cases of physical examination. In addition, with the support of the Rare Disease Clinical Research Special Fund led by Prof. Zhang Shuyang, the research team is working with multiple domestic research institutes in China to map the geographical distribution of common mutations of ALDH2 in the Chinese population. Another ongoing task is to apply spatiotemporal single-cell genomics to develop biomarkers and potential new drugs based on the existing results at the level of single nucleated cells in kidney disease patients.
Translated by Liu Haiyan
Edited by Chen Gang and Wang Yao
